Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD - PubMed (original) (raw)
Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD
Sandhya Xavier et al. J Am Soc Nephrol. 2015 Apr.
Abstract
Excessive TGF-β signaling in epithelial cells, pericytes, or fibroblasts has been implicated in CKD. This list has recently been joined by endothelial cells (ECs) undergoing mesenchymal transition. Although several studies focused on the effects of ablating epithelial or fibroblast TGF-β signaling on development of fibrosis, there is a lack of information on ablating TGF-β signaling in the endothelium because this ablation causes embryonic lethality. We generated endothelium-specific heterozygous TGF-β receptor knockout (TβRII(endo+/-)) mice to explore whether curtailed TGF-β signaling significantly modifies nephrosclerosis. These mice developed normally, but showed enhanced angiogenic potential compared with TβRII(endo+/+) mice under basal conditions. After induction of folic acid nephropathy or unilateral ureteral obstruction, TβRII(endo+/-) mice exhibited less tubulointerstitial fibrosis, enhanced preservation of renal microvasculature, improvement in renal blood flow, and less tissue hypoxia than TβRII(endo+/+) counterparts. In addition, partial deletion of TβRII in the endothelium reduced endothelial-to-mesenchymal transition (EndoMT). TGF-β-induced canonical Smad2 signaling was reduced in TβRII(+/-) ECs; however, activin receptor-like kinase 1 (ALK1)-mediated Smad1/5 phosphorylation in TβRII(+/-) ECs remained unaffected. Furthermore, the S-endoglin/L-endoglin mRNA expression ratio was significantly lower in TβRII(+/-) ECs compared with TβRII(+/+) ECs. These observations support the hypothesis that EndoMT contributes to renal fibrosis and curtailing endothelial TGF-β signals favors Smad1/5 proangiogenic programs and dictates increased angiogenic responses. Our data implicate endothelial TGF-β signaling and EndoMT in regulating angiogenic and fibrotic responses to injury.
Keywords: ALK1; Endoglin; Smad1/5; TGF-β receptor type II; microvasculature; renal fibrosis.
Copyright © 2015 by the American Society of Nephrology.
Figures
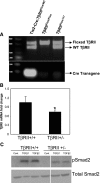
Figure 1.
Characterization of endothelial T_β_RII heterozygote mice. (A) Genotyping of pups from TGF-_β_RIIFlox/Flox and Tie2-Cre: TGF-_β_RIIFlox/WT matings. Tail PCR genotyping analysis. The top panel shows detection of floxed and WT fragments. The bottom panel shows detection of Cre-transgene. (B) Real-time PCR analysis for T_β_RII mRNA expression in kidney ECs isolated from T_β_RIIendo+/+ and T_β_RIIendo+/− mice (_n_=4). *P<0.05, T_β_RII+/+ versus T_β_RII+/−. (C) Western blot for Smad2 signaling in kidney ECs. WT, wild type; Cont, control.
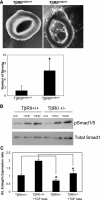
Figure 2.
Functional characterization of T_β_RII heterozygote mice. (A) Images and quantification of ex vivo angiogenesis assays in three-dimensional matrigel using explant cultures of aortic ring segments at day 6 from T_β_RIIendo+/+ and T_β_RIIendo+/− mice (_n_=5). (B) Western blot for Smad1/5 signaling in kidney ECs isolated from T_β_RIIendo+/+ and T_β_RIIendo+/− mice. (C) Endoglin S/L mRNA expression ratios in kidney ECs. *P<0.05, T_β_RII+/+ versus T_β_RII+/− and TGF-_β_1 treated T_β_RII+/+ versus T_β_RII+/−. Cont, control.
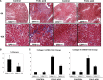
Figure 3.
Fibrosis is reduced in T_β_RIIendo+/− mice with FA toxicity. (A) Representative images of Masson’s trichrome–stained kidney sections from 12-week-old T_β_RIIendo+/+ and T_β_RIIendo+/− mice treated with vehicle or FA 6 weeks after injection (_n_=5). (B) Quantification of fibrotic area (color quantification method). (C) Expression of collagen I and collagen III examined by quantitative real-time PCR. *P<0.05, treated T_β_RIIendo+/+ versus treated T_β_RIIendo+/−. Original magnification, ×4 and ×10.
Figure 4.
Fibrosis is reduced in T_β_RIIendo+/− UUO mice. (A) Representative images of Masson’s trichrome–stained UUO and contralateral kidney sections from T_β_RIIendo+/+ and T_β_RIIendo+/− mice (_n_=4). (B) Quantification of fibrotic area (color quantification method) *P<0.01, T_β_RIIendo+/+ UUO versus T_β_RIIendo+/− UUO. (C) Representative images for _α_-SMA staining in UUO and contralateral kidney sections from T_β_RIIendo+/+ and T_β_RIIendo+/− UUO mice. Original magnification, ×4 and ×10.
Figure 5.
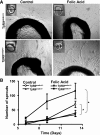
Ex vivo angiogenesis assays in mice with FA nephrotoxicity. (A) Representative images of sprouting capillary cords in aortic explants at day 13. (B) Quantitative angiogenesis analysis in T_β_RIIendo+/+ or T_β_RIIendo+/− vehicle or FA-treated animals. *P<0.01 untreated versus treated T_β_RIIendo+/+ or untreated versus treated T_β_RIIendo+/−.
Figure 6.
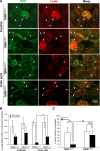
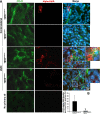
Microvascular density (CD31) and patency (lectin) in mice with FA nephrotoxicity. (A) Representative images for CD31 (green) and lectin (red) staining (_n_=5). (B) Average lengths (in micrometers) of CD31- or lectin-positive peritubular capillaries per image. (C) Ratios of average lengths of lectin- to CD31-positive capillaries (percentage) per image. *P<0.01, treated T_β_RIIendo+/+ versus T_β_RIIendo+/− lectin-positive capillary length; **P<0.001, control versus FA-treated T_β_RIIendo+/+ mice; #P<0.01, treated T_β_RIIendo+/+ versus T_β_RIIendo+/−; N.S., _P=_NS for control versus FA-treated T_β_RIIendo+/− mice. Original magnification, ×40. Per the journal style, P values were rounded to two decimal places.
Figure 7.
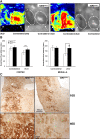
Renal blood flow measurement and pimonidazole staining in kidneys of T_β_RIIendo+/+ and T_β_RIIendo+/− UUO mice. (A) Representative image scans from laser-Doppler flowmetry analysis of contralateral and UUO kidneys from T_β_RIIendo+/+ and T_β_RIIendo+/− mice (_n_=3). (B) Quantification of cortex and medullary renal blood flow in kidneys. *P<0.001, TβRIIendo+/+ and TβRIIendo+/− UUO mice. (C) Immunohistochemical staining for pimonidazole adducts in UUO kidneys of T_β_RIIendo+/+ and T_β_RIIendo+/− mice. Original magnification, ×10 and ×40.
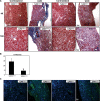
Figure 8.
EndoMT in mice with FA nephrotoxicity. (A) Representative images for CD31 (green) and _α_-SMA (red) staining (_n_=5). Wild-type control kidneys shows costaining for CD31 and _α_-SMA only in vessels. These areas were excluded during quantitative analysis. (B) Quantitative analysis of CD31 plus _α_-SMA double-positive cells in T_β_RIIendo+/+ or T_β_RIIendo+/− vehicle- or FA-treated mice. *P<0.01; #P<0.01. Original magnification, ×60.
Figure 9.
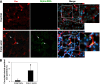
EndoMT in UUO mice. (A) Representative images for CD31 (green) and _α_-SMA (red) staining (_n_=4). No primary antibody controls from CD31 staining and _α_-SMA staining are also shown. (B) Quantitative analysis of CD31 plus _α_-SMA double-positive cells in UUO kidneys of T_β_RIIendo+/+ and T_β_RIIendo+/− mice. *P<0.01. Original magnification, ×60.
Figure 10.
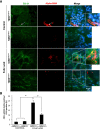
EndoMT in T_β_RIIendo+/− mice with FA nephrotoxicity. (A) Representative images for Cre (red) and _α_-SMA (green) staining (_n_=5). Arrows indicate _α_-SMA–stained areas with and without Cre costaining. (B) Quantitative analysis of Cre plus _α_-SMA double-positive cells in T_β_RIIendo+/− vehicle or FA-treated mice. *P<0.01. Original magnification, ×40.
Figure 11.
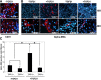
Modulation of EndoMT in T_β_RII+/+ and T_β_RII+/− kidney ECs. (A) Immunofluorescence images for CD31 or _α_-SMA in cultured T_β_RII+/+ and T_β_RII+/− kidney ECs treated with TGF-_β_1 (5 ng/ml) for 6 days (_n_=5). The nuclei are stained with 4′,6-diamidino-2-phenylindole. (B) Quantitative analysis of _α_-SMA–positive cells per 100 cells. *P<0.002. Original magnification, ×10 for _α_-SMA; ×40 for CD31 and _α_-SMA.
Comment in
- Microvascular endothelial cells poised to take center stage in experimental renal fibrosis.
Daehn I, Bottinger EP. Daehn I, et al. J Am Soc Nephrol. 2015 Apr;26(4):767-9. doi: 10.1681/ASN.2014121170. Epub 2014 Dec 22. J Am Soc Nephrol. 2015. PMID: 25535302 Free PMC article. No abstract available.
References
- Harris RC, Neilson EG: Toward a unified theory of renal progression. Annu Rev Med 57: 365–380, 2006 - PubMed
- Böttinger EP, Bitzer M: TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 - PubMed
- Kopp JB, Factor VM, Mozes M, Nagy P, Sanderson N, Böttinger EP, Klotman PE, Thorgeirsson SS: Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest 74: 991–1003, 1996 - PubMed
- Goumans MJ, Mummery C: Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44: 253–265, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK052783/DK/NIDDK NIH HHS/United States
- R01 DK045462/DK/NIDDK NIH HHS/United States
- DK54602/DK/NIDDK NIH HHS/United States
- DK052783/DK/NIDDK NIH HHS/United States
- DK45462/DK/NIDDK NIH HHS/United States
- R01 DK054602/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases