Carrier-mediated cocaine transport at the blood-brain barrier as a putative mechanism in addiction liability - PubMed (original) (raw)
Carrier-mediated cocaine transport at the blood-brain barrier as a putative mechanism in addiction liability
Hélène Chapy et al. Int J Neuropsychopharmacol. 2014.
Erratum in
- Erratum.
[No authors listed] [No authors listed] Int J Neuropsychopharmacol. 2016 Apr 27;19(10):pyw031. doi: 10.1093/ijnp/pyw031. Int J Neuropsychopharmacol. 2016. PMID: 27207904 Free PMC article. No abstract available.
Abstract
Background: The rate of entry of cocaine into the brain is a critical factor that influences neuronal plasticity and the development of cocaine addiction. Until now, passive diffusion has been considered the unique mechanism known by which cocaine crosses the blood-brain barrier.
Methods: We reassessed mechanisms of transport of cocaine at the blood-brain barrier using a human cerebral capillary endothelial cell line (hCMEC/D3) and in situ mouse carotid perfusion.
Results: Both in vivo and in vitro cocaine transport studies demonstrated the coexistence of a carrier-mediated process with passive diffusion. At pharmacological exposure level, passive diffusion of cocaine accounted for only 22.5% of the total cocaine influx in mice and 5.9% in hCMEC/D3 cells, whereas the carrier-mediated influx rate was 3.4 times greater than its passive diffusion rate in vivo. The functional identification of this carrier-mediated transport demonstrated the involvement of a proton antiporter that shared the properties of the previously characterized clonidine and nicotine transporter. The functionnal characterization suggests that the solute carrier (SLC) transporters Oct (Slc22a1-3), Mate (Slc47a1) and Octn (Slc22a4-5) are not involved in the cocaine transport in vivo and in vitro. Diphenhydramine, heroin, tramadol, cocaethylene, and norcocaine all strongly inhibited cocaine transport, unlike benzoylecgonine. Trans-stimulation studies indicated that diphenhydramine, nicotine, 3,4-methylenedioxyamphetamine (ecstasy) and the cathinone compound 3,4-methylenedioxypyrovalerone (MDPV) were also substrates of the cocaine transporter.
Conclusions: Cocaine transport at the BBB involves a proton-antiporter flux that is quantitatively much more important than its passive diffusion. The molecular identification and characterization of this transporter will provide new tools to understand its role in addictive mechanisms.
Keywords: biological transport; blood-brain barrier; cocaine; drug of abuse; pharmacokinetics..
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
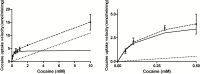
Figure 1.
Passive and carrier-mediated flux of cocaine in hCMEC/D3 cells. A, Total uptake (nmol/min/mg; dashed line) was measured in hCMEC/D3 cells and plotted against total cocaine concentration in the KH incubation buffer at pHe 7.40. The straight dotted line represents the passive transport of cocaine (K passive of 1.09±0.09 µL/min/mg at pH 7.40). The solid line represents the curve obtained by subtracting the passive flux from the total flux and fitting to the carrier-mediated Michaelis-Menten term (see Equation 7) by nonlinear least-square regression. Estimated parameters for cocaine transport in hCMEC/D3 cells are: K m, 0.123±0.023mM; V max, 4.26±0.26 nmol/min/mg. Data represent means±SD of experiments performed in triplicate. B, Total (dashed line) and individual passive (dotted line) and carrier-mediated (solid line) cocaine transport into hCMEC/D3 cells fitted according to Equation 7 for concentrations <0.5mM. Data represent means±SD of experiments performed in triplicate.
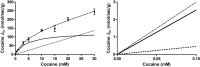
Figure 2.
Passive and carrier-mediated flux of cocaine at the mouse luminal BBB. A, Total flux (J in; nmol/sec/g; dashed line) measured in the right brain hemisphere of male Swiss mice and plotted against total cocaine concentration in the perfusion buffer (Krebs-carbonate) at pHe 7.40 (Equation 6). The straight dotted line represents the passive diffusion flux of cocaine (K passive of 4.5±1.0 µL/sec/g at pH 7.4). The solid line represents the curve obtained by subtracting the passive flux from the total flux and fitted to the carrier-mediated Michaelis-Menten term (see Equation 7) by nonlinear least-square regression. Estimated parameters for the brain transport of cocaine are: K m, 4.5±1.8mM; V max, 127.2±34.5 nmol/sec/g. Data represent means±SD of 4 to 5 mice. B, Total (dashed line), carrier-mediated (solid line), and passive (dotted line) cocaine fluxes at pharmacological concentrations at the mouse BBB, fitted according to Equation 7 for concentrations <0.1mM.
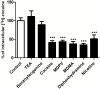
Figure 3.
_Trans_-stimulation studies of cocaine transport in hCMEC/D3 cells. hCMEC/D3 cells were loaded with [3H]-cocaine for 5 minutes and then incubated with KH buffer alone (control) or with 10 µM of unlabeled compound (TEA, benzoylecgonine, cocaine, MDPV, MDMA, diphenhydramine, or nicotine) in KH buffer. Data represent means±SD performed in quadruplicate. *** P<0.001 compared with controls.
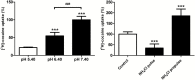
Figure 4.
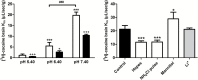
Effect of the modulation of extracellular (pHe) and intracellular (pHi) pH on cocaine transport in hCMEC/D3 cells. a, Effects of changes in the incubation buffer pHe. [3H]-Cocaine uptake was measured for 5 minutes in KH buffer adjusted to pH 5.40, 6.40, or 7.40. *** P<0.001 compared with the pH 5.4 group, and ### P<0.001 for a comparison between pH 6.40 and 7.40 (n=3–4). b, Modulation of pHi with NH4Cl. In pulse condition (cellular alkalinization): after 30 minutes of the usual preincubation, a solution (pHe 7.40) with NH4Cl (30mM) and containing [3H]-cocaine was added for 5 minutes. The NH4Cl prepulse condition (cellular acidification) was obtained by preincubating cells with the incubation buffer (pHe 7.40) plus NH4Cl (30mM) for 15 minutes. Then, the incubation medium was replaced by the usual KH buffer without NH4Cl for 5 minutes. [3H]-Cocaine uptake was measured in the usual KH incubation buffer for 5 minutes (n=3–4).
Figure 5.
Effects of changes in the vascular perfusion medium pHe, alteration of the BBB pHi, and sodium dependency of [3H]-cocaine transport at the BBB in Swiss mice. a, Effects of the Krebs-carbonate perfusion buffer at a pHe of 5.40, 6.40, or 7.40 on [3H]-cocaine brain transport (K in; µL/sec/g), with (black column) or without (white column) co-perfusion with unlabeled cocaine (10mM), measured by in situ mouse brain perfusion for 60 seconds. Data represent means±SD (n=4 mice). * P<0.05, ** P<0.01, *** P<0.001 compared with the pH 5.4 group; + P<0.05, +++ P<0.001 for comparison (Student t test) between with and without unlabeled cocaine at the same pH; and ### P<0.001 for a comparison of pH 6.40 and 7.40 without unlabeled cocaine. b, Effects of altering pHi (white columns) and removing sodium (grey column). The vascular perfusion media were Krebs-carbonate buffer plus NH4Cl (30mM; “NH4Cl pulse”), carbonate-free HEPES-buffered solution, and “mannitol” (sodium-free and chloride-free) Krebs-carbonate buffer to alter pHi (white columns). The effect of Na+-free perfusion buffer (pHe 7.40) was studied by replacing sodium with lithium (Li+; grey column). Data represent means±SD of 4 to 7 animals. * P<0.05, ** P<0.01, *** P<0.001 compared with the control group.
Similar articles
- A polyspecific drug/proton antiporter mediates diphenhydramine and clonidine transport at the mouse blood-retinal barrier.
Chapy H, André P, Declèves X, Scherrmann JM, Cisternino S. Chapy H, et al. Br J Pharmacol. 2015 Oct;172(19):4714-25. doi: 10.1111/bph.13246. Epub 2015 Aug 10. Br J Pharmacol. 2015. PMID: 26177775 Free PMC article. - Coexistence of passive and proton antiporter-mediated processes in nicotine transport at the mouse blood-brain barrier.
Cisternino S, Chapy H, André P, Smirnova M, Debray M, Scherrmann JM. Cisternino S, et al. AAPS J. 2013 Apr;15(2):299-307. doi: 10.1208/s12248-012-9434-6. Epub 2012 Dec 5. AAPS J. 2013. PMID: 23212563 Free PMC article. - Organic cation transporter 1 (OCT1) is involved in pentamidine transport at the human and mouse blood-brain barrier (BBB).
Sekhar GN, Georgian AR, Sanderson L, Vizcay-Barrena G, Brown RC, Muresan P, Fleck RA, Thomas SA. Sekhar GN, et al. PLoS One. 2017 Mar 31;12(3):e0173474. doi: 10.1371/journal.pone.0173474. eCollection 2017. PLoS One. 2017. PMID: 28362799 Free PMC article. - The blood-brain barrier choline transporter.
Geldenhuys WJ, Allen DD. Geldenhuys WJ, et al. Cent Nerv Syst Agents Med Chem. 2012 Jun;12(2):95-9. doi: 10.2174/187152412800792670. Cent Nerv Syst Agents Med Chem. 2012. PMID: 22483271 Review. - Impact of Nicotine Transport across the Blood-Brain Barrier: Carrier-Mediated Transport of Nicotine and Interaction with Central Nervous System Drugs.
Tega Y, Yamazaki Y, Akanuma SI, Kubo Y, Hosoya KI. Tega Y, et al. Biol Pharm Bull. 2018;41(9):1330-1336. doi: 10.1248/bpb.b18-00134. Biol Pharm Bull. 2018. PMID: 30175770 Review.
Cited by
- Alcohol and Cocaine Exposure Modulates ABCB1 and ABCG2 Transporters in Male Alcohol-Preferring Rats.
Hammad AM, Alasmari F, Sari Y, Scott Hall F, Tiwari AK. Hammad AM, et al. Mol Neurobiol. 2019 Mar;56(3):1921-1932. doi: 10.1007/s12035-018-1153-2. Epub 2018 Jul 6. Mol Neurobiol. 2019. PMID: 29978425 Free PMC article. - Drug Permeability: From the Blood-Brain Barrier to the Peripheral Nerve Barriers.
Sun Y, Zabihi M, Li Q, Li X, Kim BJ, Ubogu EE, Raja SN, Wesselmann U, Zhao C. Sun Y, et al. Adv Ther (Weinh). 2023 Apr;6(4):2200150. doi: 10.1002/adtp.202200150. Epub 2023 Jan 18. Adv Ther (Weinh). 2023. PMID: 37649593 Free PMC article. - The biology of ergothioneine, an antioxidant nutraceutical.
Borodina I, Kenny LC, McCarthy CM, Paramasivan K, Pretorius E, Roberts TJ, van der Hoek SA, Kell DB. Borodina I, et al. Nutr Res Rev. 2020 Dec;33(2):190-217. doi: 10.1017/S0954422419000301. Epub 2020 Feb 13. Nutr Res Rev. 2020. PMID: 32051057 Free PMC article. Review. - Generation of a Small Library of Natural Products Designed to Cover Chemical Space Inexpensively.
O'Hagan S, Kell DB. O'Hagan S, et al. Pharm Front. 2019;1(1):e190005. doi: 10.20900/pf20190005. Epub 2019 Aug 9. Pharm Front. 2019. PMID: 31485581 Free PMC article. - Importance of Toxicokinetics to Assess the Utility of Zebrafish Larvae as Model for Psychoactive Drug Screening Using Meta-Chlorophenylpiperazine (mCPP) as Example.
Kirla KT, Groh KJ, Poetzsch M, Banote RK, Stadnicka-Michalak J, Eggen RIL, Schirmer K, Kraemer T. Kirla KT, et al. Front Pharmacol. 2018 Apr 26;9:414. doi: 10.3389/fphar.2018.00414. eCollection 2018. Front Pharmacol. 2018. PMID: 29755353 Free PMC article.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. (2010). Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. - PubMed
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. (2001). Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 154:76–84. - PubMed
- Agarwal S, Uchida Y, Mittapalli RK, Sane R, Terasaki T, Elmquist WF. (2012). Quantitative proteomics of transporter expression in brain capillary endothelial cells isolated from P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp), and P-gp/Bcrp knockout mice. Drug Metab Dispos 40:1164–1169. - PMC - PubMed
- Andre P, Debray M, Scherrmann JM, Cisternino S. (2009). Clonidine transport at the mouse blood-brain barrier by a new H+ antiporter that interacts with addictive drugs. J Cereb Blood Flow Metab 29:1293–1304. - PubMed
- Andre P, Saubamea B, Cochois-Guegan V, Marie-Claire C, Cattelotte J, Smirnova M, Schinkel AH, Scherrmann JM, Cisternino S. (2012). Transport of biogenic amine neurotransmitters at the mouse blood-retina and blood-brain barriers by uptake1 and uptake2. J Cereb Blood Flow Metab 32:1989–2001. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources