Considerations and challenges in defining optimal iron utilization in hemodialysis - PubMed (original) (raw)
Review
. 2015 Jun;26(6):1238-47.
doi: 10.1681/ASN.2014090922. Epub 2014 Dec 26.
Collaborators, Affiliations
- PMID: 25542967
- PMCID: PMC4446883
- DOI: 10.1681/ASN.2014090922
Review
Considerations and challenges in defining optimal iron utilization in hemodialysis
David M Charytan et al. J Am Soc Nephrol. 2015 Jun.
Abstract
Trials raising concerns about erythropoiesis-stimulating agents, revisions to their labeling, and changes to practice guidelines and dialysis payment systems have provided strong stimuli to decrease erythropoiesis-stimulating agent use and increase intravenous iron administration in recent years. These factors have been associated with a rise in iron utilization, particularly among hemodialysis patients, and an unprecedented increase in serum ferritin concentrations. The mean serum ferritin concentration among United States dialysis patients in 2013 exceeded 800 ng/ml, with 18% of patients exceeding 1200 ng/ml. Although these changes are broad based, the wisdom of these practices is uncertain. Herein, we examine influences on and trends in intravenous iron utilization and assess the clinical trial, epidemiologic, and experimental evidence relevant to its safety and efficacy in the setting of maintenance dialysis. These data suggest a potential for harm from increasing use of parenteral iron in dialysis-dependent patients. In the absence of well powered, randomized clinical trials, available evidence will remain inadequate for making reliable conclusions about the effect of a ubiquitous therapy on mortality or other outcomes of importance to dialysis patients. Nephrology stakeholders have an urgent obligation to initiate well designed investigations of intravenous iron in order to ensure the safety of the dialysis population.
Keywords: ESRD; anemia; dialysis; erythropoietin.
Copyright © 2015 by the American Society of Nephrology.
Figures
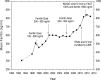
Figure 1.
Trends in mean serum ferritin over time. Data sources include the ESRD Core Indicators Project, the USRDS, the Dialysis Outcomes Practices Patterns Study, the Network 11 ELab Project, and the Medicare Clinical Performance Measure Reports.,,, IV, intravenous.
Similar articles
- When should iron supplementation in dialysis patients be avoided, minimized or withdrawn?
Rostoker G. Rostoker G. Semin Dial. 2019 Jan;32(1):22-29. doi: 10.1111/sdi.12732. Epub 2018 Jun 28. Semin Dial. 2019. PMID: 29956370 Free PMC article. - Changing patterns of anemia management in US hemodialysis patients.
Freburger JK, Ng LJ, Bradbury BD, Kshirsagar AV, Brookhart MA. Freburger JK, et al. Am J Med. 2012 Sep;125(9):906-14.e9. doi: 10.1016/j.amjmed.2012.03.011. Am J Med. 2012. PMID: 22938926 - Updating practices in an evolving IV iron and anemia environment: practical solutions.
Amerling R, Easom A, Juergensen P. Amerling R, et al. Nephrol Nurs J. 2007 Sep-Oct;34(5):533-41; quiz 542-3. Nephrol Nurs J. 2007. PMID: 18041456 Review. - Adverse events in chronic hemodialysis patients receiving intravenous iron dextran--a comparison of two products.
McCarthy JT, Regnier CE, Loebertmann CL, Bergstralh EJ. McCarthy JT, et al. Am J Nephrol. 2000 Nov-Dec;20(6):455-62. doi: 10.1159/000046199. Am J Nephrol. 2000. PMID: 11146312 Clinical Trial. - Iron Treatment Strategies in Dialysis-Dependent CKD.
Pandey R, Daloul R, Coyne DW. Pandey R, et al. Semin Nephrol. 2016 Mar;36(2):105-11. doi: 10.1016/j.semnephrol.2016.02.004. Semin Nephrol. 2016. PMID: 27236131 Review.
Cited by
- Evaluation of the Cost of a High-Dose Intravenous Iron Protocol in a Regional Hemodialysis Program: Research Letter.
Papini A, Manns BJ, Elliott MJ. Papini A, et al. Can J Kidney Health Dis. 2021 Dec 8;8:20543581211063984. doi: 10.1177/20543581211063984. eCollection 2021. Can J Kidney Health Dis. 2021. PMID: 35186304 Free PMC article. - Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease.
Ueda N, Takasawa K. Ueda N, et al. Nutrients. 2018 Aug 27;10(9):1173. doi: 10.3390/nu10091173. Nutrients. 2018. PMID: 30150549 Free PMC article. Review. - New options for the anemia of chronic kidney disease.
Coyne DW, Goldsmith D, Macdougall IC. Coyne DW, et al. Kidney Int Suppl (2011). 2017 Dec;7(3):157-163. doi: 10.1016/j.kisu.2017.09.002. Epub 2017 Nov 17. Kidney Int Suppl (2011). 2017. PMID: 30675430 Free PMC article. Review. - Combination with intravenous iron supplementation or doubling erythropoietin dose for patients with chemotherapy-induced anaemia inadequately responsive to initial erythropoietin treatment alone: study protocol for a randomised controlled trial.
Chen L, Jiang H, Gao W, Tu Y, Zhou Y, Li X, Zhu Z, Jiang Q, Zhan H, Yu J, Fu C, Gao Y. Chen L, et al. BMJ Open. 2016 Oct 7;6(10):e012231. doi: 10.1136/bmjopen-2016-012231. BMJ Open. 2016. PMID: 27855097 Free PMC article. Clinical Trial. - Coronary artery bypass grafting vs. percutaneous coronary intervention in coronary artery disease patients with advanced chronic kidney disease: A Chinese single-center study.
Li Y, Hou X, Xu X, Huang Z, Liu T, Xu S, Rui H, Zheng J, Dong R. Li Y, et al. Front Surg. 2023 Jan 20;9:1042186. doi: 10.3389/fsurg.2022.1042186. eCollection 2022. Front Surg. 2023. PMID: 36743894 Free PMC article.
References
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 - PubMed
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 - PubMed
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 - PubMed
- Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A, CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 - PubMed
- US Food and Drug Administration: US FDA drug safety communication: Modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Accessed June 11, 2014
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK091288/DK/NIDDK NIH HHS/United States
- R01-DK091288/DK/NIDDK NIH HHS/United States
- DK096189/DK/NIDDK NIH HHS/United States
- HL11831402/HL/NHLBI NIH HHS/United States
- R01 DK096920/DK/NIDDK NIH HHS/United States
- DK100772/DK/NIDDK NIH HHS/United States
- U01FD004889/FD/FDA HHS/United States
- U01 DK096189/DK/NIDDK NIH HHS/United States
- R21 DK100772/DK/NIDDK NIH HHS/United States
- R01 HL118314/HL/NHLBI NIH HHS/United States
- U01 DK102163/DK/NIDDK NIH HHS/United States
- U01 FD004889/FD/FDA HHS/United States
- R01-DK096920/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases