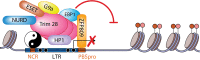Retroviral transcriptional regulation and embryonic stem cells: war and peace - PubMed (original) (raw)
Review
Retroviral transcriptional regulation and embryonic stem cells: war and peace
Sharon Schlesinger et al. Mol Cell Biol. 2015 Mar.
Abstract
Retroviruses have evolved complex transcriptional enhancers and promoters that allow their replication in a wide range of tissue and cell types. Embryonic stem (ES) cells, however, characteristically suppress transcription of proviruses formed after infection by exogenous retroviruses and also of most members of the vast array of endogenous retroviruses in the genome. These cells have unusual profiles of transcribed genes and are poised to make rapid changes in those profiles upon induction of differentiation. Many of the transcription factors in ES cells control both host and retroviral genes coordinately, such that retroviral expression patterns can serve as markers of ES cell pluripotency. This overlap is not coincidental; retrovirus-derived regulatory sequences are often used to control cellular genes important for pluripotency. These sequences specify the temporal control and perhaps "noisy" control of cellular genes that direct proper cell gene expression in primitive cells and their differentiating progeny. The evidence suggests that the viral elements have been domesticated for host needs, reflecting the wide-ranging exploitation of any and all available DNA sequences in assembling regulatory networks.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
FIG 1
Transcriptional regulation of the integrated provirus in embryos and embryonic cells. Trim28 binding to the DNA binding proteins ZFP809 (14) and YY1 (17), as well as to EBP1 (21) and HP1 (20), results in epigenetic silencing of the newly integrated or endogenous provirus elements. The silencing is mediated by Trim28 recruitment of many factors involved in transcriptional silencing and heterochromatin formation, including the histone methyltransferases ESET (22) and G9a (34) and the NuRD histone deacetylase complex (92). These histone modifiers are responsible for the trimethylation of H3K9 (H3K9Me3), H4K20 (H4K20Me3) (22), and H3K27 (H3K27me3) (24) (red and pink circles) histone tails as well as for subsequent DNA methylation (93). NCR, noncoding region.
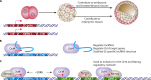
FIG 2
Examples of domestication of ERV sequences by mouse and human embryonic cells. (A) MERV-L elements and their remnant “solo” long terminal repeats (LTRs) have been coopted to participate in gene regulatory networks by serving as primary or alternative promoters of nearby genes (31). A subset of mouse embryonic stem cells expresses MERV-L LTR-driven chimeric transcripts, which correlates with increased potency (46). (B) HERV-H interacts with Oct4 to promote the enhancer activities of LTR7 and nearby regions and to drive the expression of neighboring lncRNAs and protein-coding genes essential to hES cell identity (59, 61, 63). (C) ERV1 elements in the human and mouse genomes carry transcription factor-binding sites for Oct4 and Nanog, which can regulate genes that form the pluripotency network near insertion sites, leading to novel regulatory patterns in evolving mammals (9, 52).
Similar articles
- How to tame an endogenous retrovirus: HERVH and the evolution of human pluripotency.
Römer C, Singh M, Hurst LD, Izsvák Z. Römer C, et al. Curr Opin Virol. 2017 Aug;25:49-58. doi: 10.1016/j.coviro.2017.07.001. Epub 2017 Jul 24. Curr Opin Virol. 2017. PMID: 28750248 Review. - Embryonic stem cell potency fluctuates with endogenous retrovirus activity.
Macfarlan TS, Gifford WD, Driscoll S, Lettieri K, Rowe HM, Bonanomi D, Firth A, Singer O, Trono D, Pfaff SL. Macfarlan TS, et al. Nature. 2012 Jul 5;487(7405):57-63. doi: 10.1038/nature11244. Nature. 2012. PMID: 22722858 Free PMC article. - Histone H3.3 is required for endogenous retroviral element silencing in embryonic stem cells.
Elsässer SJ, Noh KM, Diaz N, Allis CD, Banaszynski LA. Elsässer SJ, et al. Nature. 2015 Jun 11;522(7555):240-244. doi: 10.1038/nature14345. Epub 2015 May 4. Nature. 2015. PMID: 25938714 Free PMC article. - The endogenous retrovirus ENS-1 provides active binding sites for transcription factors in embryonic stem cells that specify extra embryonic tissue.
Mey A, Acloque H, Lerat E, Gounel S, Tribollet V, Blanc S, Curton D, Birot AM, Nieto MA, Samarut J. Mey A, et al. Retrovirology. 2012 Mar 15;9:21. doi: 10.1186/1742-4690-9-21. Retrovirology. 2012. PMID: 22420414 Free PMC article. - Human endogenous retroviruses: friend or foe?
Weiss RA. Weiss RA. APMIS. 2016 Jan-Feb;124(1-2):4-10. doi: 10.1111/apm.12476. APMIS. 2016. PMID: 26818257 Review.
Cited by
- Effective and stable gene transduction in rhesus macaque iPSCs capable of T-lineage differentiation utilizing the piggyBac system.
Tanaka M, Iwamoto Y, Wang B, Imai E, Yoshida M, Iriguchi S, Kaneko S. Tanaka M, et al. Regen Ther. 2024 Mar 18;27:104-111. doi: 10.1016/j.reth.2024.03.002. eCollection 2024 Dec. Regen Ther. 2024. PMID: 38545443 Free PMC article. - T-bet+ B cells are activated by and control endogenous retroviruses through TLR-dependent mechanisms.
Rauch E, Amendt T, Lopez Krol A, Lang FB, Linse V, Hohmann M, Keim AC, Kreutzer S, Kawengian K, Buchholz M, Duschner P, Grauer S, Schnierle B, Ruhl A, Burtscher I, Dehnert S, Kuria C, Kupke A, Paul S, Liehr T, Lechner M, Schnare M, Kaufmann A, Huber M, Winkler TH, Bauer S, Yu P. Rauch E, et al. Nat Commun. 2024 Feb 9;15(1):1229. doi: 10.1038/s41467-024-45201-6. Nat Commun. 2024. PMID: 38336876 Free PMC article. - Advances in Genetic Reprogramming: Prospects from Developmental Biology to Regenerative Medicine.
Dhanjal DS, Singh R, Sharma V, Nepovimova E, Adam V, Kuca K, Chopra C. Dhanjal DS, et al. Curr Med Chem. 2024;31(13):1646-1690. doi: 10.2174/0929867330666230503144619. Curr Med Chem. 2024. PMID: 37138422 Review. - Rif1 interacts with non-canonical polycomb repressive complex PRC1.6 to regulate mouse embryonic stem cells fate potential.
Li L, Li P, Chen J, Li L, Shen Y, Zhu Y, Liu J, Lv L, Mao S, Chen F, Hu G, Yuan K. Li L, et al. Cell Regen. 2022 Aug 2;11(1):25. doi: 10.1186/s13619-022-00124-9. Cell Regen. 2022. PMID: 35915272 Free PMC article. - A novel class III endogenous retrovirus with a class I envelope gene in African frogs with an intact genome and developmentally regulated transcripts in Xenopus tropicalis.
Yedavalli VRK, Patil A, Parrish J, Kozak CA. Yedavalli VRK, et al. Retrovirology. 2021 Jul 14;18(1):20. doi: 10.1186/s12977-021-00564-2. Retrovirology. 2021. PMID: 34261506 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources