Cdc45 (cell division cycle protein 45) guards the gate of the Eukaryote Replisome helicase stabilizing leading strand engagement - PubMed (original) (raw)
Cdc45 (cell division cycle protein 45) guards the gate of the Eukaryote Replisome helicase stabilizing leading strand engagement
Tatjana Petojevic et al. Proc Natl Acad Sci U S A. 2015.
Abstract
DNA replication licensing is now understood to be the pathway that leads to the assembly of double hexamers of minichromosome maintenance (Mcm2-7) at origin sites. Cell division control protein 45 (Cdc45) and GINS proteins activate the latent Mcm2-7 helicase by inducing allosteric changes through binding, forming a Cdc45/Mcm2-7/GINS (CMG) complex that is competent to unwind duplex DNA. The CMG has an active gate between subunits Mcm2 and Mcm5 that opens and closes in response to nucleotide binding. The consequences of inappropriate Mcm2/5 gate actuation and the role of a side channel formed between GINS/Cdc45 and the outer edge of the Mcm2-7 ring for unwinding have remained unexplored. Here we uncover a novel function for Cdc45. Cross-linking studies trace the path of the DNA with the CMG complex at a fork junction between duplex and single strands with the bound CMG in an open or closed gate conformation. In the closed state, the lagging strand does not pass through the side channel, but in the open state, the leading strand surprisingly interacts with Cdc45. Mutations in the recombination protein J fold of Cdc45 that ablate this interaction diminish helicase activity. These data indicate that Cdc45 serves as a shield to guard against occasional slippage of the leading strand from the core channel.
Keywords: CMG helicase; Cdc45; DNA replication; Mcm2/5 gate; RecJ fold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
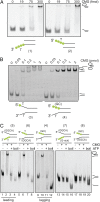
Poly-T stretches help orient CMG binding to forks with single strands. (A) CMG binding to DNA was titrated with fluorescein-labeled DNA substrates (indicated by green dots). The substrates used are diagrammed and have single-strand overhangs from a duplex with poly-T with either a 3′ or 5′ extension. CMG was added to 50 fmol of DNA in a range from 0, 19, 75 and 300 fmol of protein. In this figure and all others, the numbers under the diagrams of the probes refer to Table S1, where the exact sequences are given. (B) Indicated amounts of CMG were incubated with 100 fmol of DNA substrates with 3′+5′ extensions of poly-T tracks (“fork” substrate) or 3′ poly-T+ 5′ GC (leading strand substrate) extensions. The fork substrate displays a second supershifted species in the EMSA, which is not present with the leading strand substrate. Signals were detected by fluorescence, and positions of free forked DNA substrates and CMG-bound substrates are indicated on the side. (C) DNA binding and helicase activities of CMG were tested using five forked DNA substrates with different nucleotide sequences on the 3′ and 5′ arms (see Table S1 for DNA sequences). For each substrate, 300 fmol of CMG were bound to 50 fmol of DNA in the presence of ATPγS (lanes 2, 6, 10, 14, and 18). For helicase activity, a 100-fold excess of ATP was added and unwinding was detected (lanes 3, 7, 11, 15, and 19). Positions of free DNA substrates, CMG-bound substrates, and displaced substrates are indicated on the side. Free (no protein, lanes 1, 5, 9, 13, and 17) and “boiled” substrates (lanes 4, 8, 12, 16, and 20) are shown for each of the used substrates.
Fig. 2.
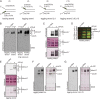
Cdc45 in the CMG contacts single strands of a fork only in the APO state. (A) Schematic for leading and lagging strand DNA substrates used in UV-induced cross-linking assays. The sequence composition of the two single-strand arms and the position of the fluorescein-conjugated nucleotides (green dots) are indicated, and sequences are provided in Table S1. (B, Left) Immunoblot of leading and lagging DNA substrates cross-linked to wild-type CMG in the absence and presence of ATPγS. We used 200 fmol of protein and 200 fmol of DNA. After UV cross-linking and nuclease digestion as described, proteins were separated by SDS/PAGE (10%) and transferred to support for Western blotting. The immunoblot was probed with monoclonal α-fluorescein antibody to detect cross-linked nucleotide–protein bands. The long exposure (Right, ∼6× longer than short exposure) shows that Cdc45 cross-links to the leading strand substrate in the absence of ATPγS. GINS proteins do not cross-link to either substrate. Protein bands were assigned by alignment to the SYPRO red-stained membrane as shown in Fig. S2_A_. (C, Top) Mcm2–7 part of the immunoblot when the leading strand DNA substrate is cross-linked to wild-type CMG. (Middle) The positions of all Mcm2–7 proteins are indicated on the side and were aligned to the gold-stained membrane, to which the proteins were transferred. (Bottom) Protein bands were identified by overlay of the Western signal onto the gold-stained membrane. In this and subsequent panels, proteins were separated by SDS/PAGE (8%). (D) Mcm2–7 part of the SDS/PAGE (8%) when the leading strand DNA substrate is cross-linked to wild-type CMG with MBP–Mcm3 and mCherry–Mcm5. Overlay of emission signals from SYPRO red-stained protein bands at 605 nm (red) and at 532 nm to visualize the fluorescein–nucleotide:protein bands (green) is shown. (E) Mcm2–7 part of the immunoblot when the lagging strand DNA substrate is cross-linked to wild-type CMG (Top). Same arrangement as shown under C. (F) Immunoblot shows proteins of the wild-type CMG, to which nucleotides were cross-linked using the lagging strand 2,4 DNA substrate. Overlay of gold-stained protein bands and Western blot signals of the nucleotide–protein bands is shown on the right. We used 400 fmol of protein and 400 fmol of fork DNA. (G) Immunoblot shows proteins of the wild-type CMG, to which nucleotides were cross-linked using the lagging strand 3,6,9,15 DNA substrate. We used 400 fmol of protein and 400 fmol of DNA. Overlay of gold-stained protein bands and Western blot signals of the nucleotide–protein bands is shown on the right. No significant cross-linking of Cdc45 above the background level is observed.
Fig. 3.
Mutations in Mcm2–7 affect the DNA contacts of Cdc45 in the CMG complex. (A) EMSA of wild-type CMG and CMG complex with mutations in the PS1 β-hairpin of all six Mcm2–7 proteins (named 6xPS1). “0” shows the control lane without protein. All lanes contained 100 fmol of fluorescein-labeled forked DNA substrate (3′+5′ poly-T, fork) and where indicated 100 fmol of purified CMG protein in the presence of 10 μM of ATPγS. Reactions were separated on a native TBE (Tris/Borate/EDTA) polyacrylamide gel (4%). Positions of free and CMG-bound substrates are indicated on the side. (B) Helicase activity assays. Positions of circular substrate and the single-strand oligonucleotide released from the M13 are indicated on the side. Two concentrations of CMG (25 and 200 fmol) were added to 1 fmol of radiolabeled substrate and 300 μM of ATP. Products were separated by TBE–PAGE (8%). 0 and boil are substrate controls without protein or with boiled substrate, respectively, and an autoradiogram of the reaction products is shown. (C) Immunoblot shows proteins of the 6xPS1–CMG complex that were cross-linked to the leading strand substrate under UV and treated with nuclease as described. Proteins were separated by SDS/PAGE (8%). Short (Top) and long (Bottom, ∼6 times longer) exposure times are shown. (D) Immunoblot shows proteins of the 6xPS1 CMG complex that were cross-linked to the lagging strand 2,4 DNA substrate. Reaction products were separated by SDS/PAGE (10%). (E, Left) EMSA with wild-type CMG and 6xEXT CMG complex. 0 shows the control lane without protein. All reactions had 100 fmol of leading strand substrate and 100 fmol of purified CMG protein in the presence of 10 μM of ATPγS. (Right) Helicase activity assay with leading strand substrate. After initial binding of CMG to DNA in the presence of 10 μM of ATPγS, unwinding was initiated by addition of 300 μM of ATP. Positions of free DNA and displaced strand are shown on the right. Quantifications of two independent series of both EMSA and helicase activity assay are shown below. (F) Immunoblot shows proteins of the 6xEXT–CMG complex that were cross-linked to the leading and lagging strand substrates. Reaction products were separated by SDS/PAGE (8%). For the leading strand, a longer exposure is shown on the bottom to visualize Cdc45. (G) Summary of the cross-linking results with various DNA substrates in the apo and ATPγS-bound states of CMG (subunits of complex are identified). The different substrates are color coded and listed below. The change from small to large circles indicates a switch from a cross-linking signal with the different conditions of the assays. In the apo state, Cdc45 cross-links to the leading strand, whereas to the lagging strand only right at the ssDNA–dsDNA junction. When a nucleotide is bound, the Mcm2–7 proteins cross-link to the leading strand substrate likely through the central channel, whereas the interactions with the lagging strand must reside on the exterior surface.
Fig. 4.
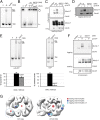
Specific Cdc45 residues contact the leading strand within the CMG complex. (A) Ribbon representation of the _N_- and C-terminal Cdc45 homology models. The T. thermophilus RecJ–exonuclease scaffold is shown as transparent with the C-terminal lobe of Cdc45 modeled in cyan above the silhouetted ribbon. The _N_-terminal domain of Cdc45 is shown in magenta. (B) Sequence alignment of Cdc45 proteins in the domain containing the two putative DNA binding residues K363 and R419 (red). Asterisks show conserved residues and dots similar residues: H. sapiens, X. laevis, M. musculus, and D. melanogaster. (C) EMSA comparing wild-type and Cdc45–mutant CMG complexes. 0 shows a control lane with substrate alone; all other lanes show 100 fmol of 3′+5′ poly-T fork with 100 fmol of CMG protein in the presence of 10 μM of ATPγS. 2x indicates mutant complexes harboring alanine substitution changes in place of K363 and R419. DNA·protein complexes were separated from the duplex on a native TBE gel (4%). (D) Immunoblot shows proteins of CMG complexes (200 fmol) with mutations in Cdc45 that were cross-linked to the leading strand DNA substrate (200 fmol). The long exposure is shown on the bottom to visualize Cdc45. (E) Immunoblot of cross-linked CMG proteins with mutations in Cdc45 (400 fmol) to the lagging strand 2,4 DNA substrate (400 fmol). (F) Quantification of CMG helicase activity assay on a circular DNA substrate. Two protein amounts (50 and 200 fmol) were assayed, and average values with SDs for six independent series are given.
Fig. 5.
Cdc45 loop affects the helicase activity of CMG. (A) The helix-loop-helix motif, in which R419 is embedded in DmCdc45. All residues highlighted in red were changed to alanine (5A mutant). (B) EMSA assay of a protein titration of wild-type and Cdc45 loop mutant (5A) CMG. 0 shows the control lane with substrate alone, and all other lanes contain 100 fmol of 3′+5′ poly-T fork with 100 fmol of CMG with 10 μM of ATPγS. Reaction products were separated on a native TBE gel (5%). (C) Immunoblot shows proteins of wild-type and Cdc45-5A mutant CMG complexes (200 fmol) that were cross-linked to the leading strand DNA substrate (200 fmol). The long exposure is shown on the bottom. (D) Immunoblot of cross-linked wild-type and Cdc45-5A mutant CMG complexes (400 fmol) to the lagging strand 2,4 DNA substrate (400 fmol). (E) Helicase activity assay on a circular DNA substrate titrating CMG proteins (30, 100, and 300 fmol) in the presence of 300 μM of ATP. Reaction products were separated by TBE–PAGE (8%). Quantification of the helicase activity is shown below with average values and SD from two independent series.
Fig. 6.
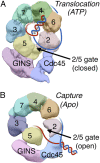
Model. Shown are AAA+ views of the CMG–EM (A) in the presence of ATPγS (“Translocation”) where the model used here are the volumes shown in Costa et al. (2014) (20) and (B) in the absence of nucleotide (“Capture”) with the model from Costa et al. (2011) (18). The leading and the lagging strands are color coded with red and blue, respectively. The model and data that support the depicted switch in DNA strand placements are derived from cross-linking and mutational data as well as structural considerations as discussed in the text.
Similar articles
- Dpb11 protein helps control assembly of the Cdc45·Mcm2-7·GINS replication fork helicase.
Dhingra N, Bruck I, Smith S, Ning B, Kaplan DL. Dhingra N, et al. J Biol Chem. 2015 Mar 20;290(12):7586-601. doi: 10.1074/jbc.M115.640383. Epub 2015 Feb 6. J Biol Chem. 2015. PMID: 25659432 Free PMC article. - The structural basis for MCM2-7 helicase activation by GINS and Cdc45.
Costa A, Ilves I, Tamberg N, Petojevic T, Nogales E, Botchan MR, Berger JM. Costa A, et al. Nat Struct Mol Biol. 2011 Apr;18(4):471-7. doi: 10.1038/nsmb.2004. Epub 2011 Mar 6. Nat Struct Mol Biol. 2011. PMID: 21378962 Free PMC article. - Mcm10 coordinates the timely assembly and activation of the replication fork helicase.
Perez-Arnaiz P, Bruck I, Kaplan DL. Perez-Arnaiz P, et al. Nucleic Acids Res. 2016 Jan 8;44(1):315-29. doi: 10.1093/nar/gkv1260. Epub 2015 Nov 17. Nucleic Acids Res. 2016. PMID: 26582917 Free PMC article. - The Eukaryotic CMG Helicase at the Replication Fork: Emerging Architecture Reveals an Unexpected Mechanism.
Li H, O'Donnell ME. Li H, et al. Bioessays. 2018 Mar;40(3):10.1002/bies.201700208. doi: 10.1002/bies.201700208. Epub 2018 Feb 6. Bioessays. 2018. PMID: 29405332 Free PMC article. Review. - The eukaryotic CMG helicase pumpjack and integration into the replisome.
Sun J, Yuan Z, Georgescu R, Li H, O'Donnell M. Sun J, et al. Nucleus. 2016 Apr 25;7(2):146-54. doi: 10.1080/19491034.2016.1174800. Nucleus. 2016. PMID: 27310307 Free PMC article. Review.
Cited by
- Visfatin Affects the Transcriptome of Porcine Luteal Cells during Early Pregnancy.
Kopij G, Kiezun M, Dobrzyn K, Zaobidna E, Zarzecka B, Rak A, Kaminski T, Kaminska B, Smolinska N. Kopij G, et al. Int J Mol Sci. 2024 Feb 16;25(4):2339. doi: 10.3390/ijms25042339. Int J Mol Sci. 2024. PMID: 38397019 Free PMC article. - Identification of key biomarker genes in liver hepatocellular carcinoma and kidney renal clear cell carcinoma progression: A shared high-throughput screening and molecular docking method with potentials for targeted therapeutic interventions.
Tasneem M, Gupta SD, Ahmed Jony MJ, Minkara M, Dey RK, Ferdoush J. Tasneem M, et al. J Genet Eng Biotechnol. 2025 Jun;23(2):100497. doi: 10.1016/j.jgeb.2025.100497. Epub 2025 Apr 22. J Genet Eng Biotechnol. 2025. PMID: 40390492 Free PMC article. - DNA translocation mechanism of the MCM complex and implications for replication initiation.
Meagher M, Epling LB, Enemark EJ. Meagher M, et al. Nat Commun. 2019 Jul 15;10(1):3117. doi: 10.1038/s41467-019-11074-3. Nat Commun. 2019. PMID: 31308367 Free PMC article. - CryoEM structures of human CMG-ATPγS-DNA and CMG-AND-1 complexes.
Rzechorzek NJ, Hardwick SW, Jatikusumo VA, Chirgadze DY, Pellegrini L. Rzechorzek NJ, et al. Nucleic Acids Res. 2020 Jul 9;48(12):6980-6995. doi: 10.1093/nar/gkaa429. Nucleic Acids Res. 2020. PMID: 32453425 Free PMC article. - Episomal and chromosomal DNA replication and recombination in Entamoeba histolytica.
Bhattacharya S. Bhattacharya S. Front Mol Biosci. 2023 Jun 8;10:1212082. doi: 10.3389/fmolb.2023.1212082. eCollection 2023. Front Mol Biosci. 2023. PMID: 37363402 Free PMC article. Review.
References
- Yardimci H, Walter JC. Prereplication-complex formation: A molecular double take? Nat Struct Mol Biol. 2014;21(1):20–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM071747/GM/NIGMS NIH HHS/United States
- GM821972/GM/NIGMS NIH HHS/United States
- CA R37-30490/CA/NCI NIH HHS/United States
- 15852/CRUK_/Cancer Research UK/United Kingdom
- GM071747/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous