Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth - PubMed (original) (raw)
Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth
S Coppenhagen-Glazer et al. Infect Immun. 2015 Mar.
Abstract
Fusobacterium nucleatum is a common oral anaerobe involved in periodontitis that is known to translocate and cause intrauterine infections. In the oral environment, F. nucleatum adheres to a large diversity of species, facilitating their colonization and creating biological bridges that stabilize the multispecies dental biofilm. Many of these interactions (called coadherences or coaggregations) are galactose sensitive. Galactose-sensitive interactions are also involved in the binding of F. nucleatum to host cells. Hemagglutination of some F. nucleatum strains is also galactose sensitive, suggesting that a single galactose-sensitive adhesin might mediate the interaction of fusobacteria with many partners and targets. In order to identify the fusobacterial galactose-sensitive adhesin, a system for transposon mutagenesis in fusobacteria was created. The mutant library was screened for hemagglutination deficiency, and three clones were isolated. All three clones were found to harbor the transposon in the gene coding for the Fap2 outer membrane autotransporter. The three fap2 mutants failed to show galactose-inhibitable coaggregation with Porphyromonas gingivalis and were defective in cell binding. A fap2 mutant also showed a 2-log reduction in murine placental colonization compared to that of the wild type. Our results suggest that Fap2 is a galactose-sensitive hemagglutinin and adhesin that is likely to play a role in the virulence of fusobacteria.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
FIG 1
F. nucleatum ATCC 23726 hemagglutination is inhibited by
d
-galactose but not by
l
-arginine. (A) Bacteria incubated with 2% sheep red blood cells (RBC) in the absence and presence of 6 mM
d
-galactose (Gal) or 50 mM
l
-arginine (Arg). (B) Erythrocytes incubated without bacteria in the absence and presence of inhibitors. Precipitation of the red blood cells (seen as a red dot) represents a lack of hemagglutination.
FIG 2
Isolation of F. nucleatum mutants deficient in hemagglutination. (A) Microtiter plate screening for library clones defective in hemagglutination. (B) Hemagglutination assay with 2-fold dilutions of the hemagglutination-deficient mutants (D22, K25, and K50) compared to wild-type F. nucleatum ATCC 23726 and the randomly selected transposon insertion controls: K151, K174, and J78.
FIG 3
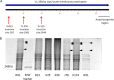
All three hemagglutination-deficient mutants are defective in Fap2. (A) Locations of transposon insertions in the fap2 genes of three hemagglutination-deficient mutants. (B) SDS-PAGE demonstrating the absence of the Fap2 protein bands in membrane proteins extracted from all three hemagglutination-deficient mutants compared to the wild type and the randomly selected mutants J78 and K174. The clear band seen in the MW marker lane indicates 245 kDa.
FIG 4
All three hemagglutination-deficient mutants fail to coaggregate with P. gingivalis. F. nucleatum and P. gingivalis were brought to an OD600 of 1 in coaggregation buffer mixed and incubated in a glass tube at room temperature for 30 min. Coaggregation (pellet), which is indicated with a red arrow, is absent in the presence of 60 mM
d
-galactose (gal) (A) and with the hemagglutinin mutants D22, K25, and K50 compared to the wild type and the randomly selected mutant J78 (B). (C and D) Coaggregation was quantified as described in Materials and Methods. All three hemagglutination-deficient mutants (but not the randomly selected mutant J78) failed to coaggregate with P. gingivalis but not with S. sanguinis. **, P < 0.01 compared to the wild-type control. ns, not significant.
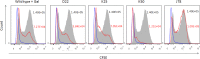
FIG 5
All three hemagglutination-deficient mutants are impaired in binding to mammalian cells. Shown are the results of fluorescence-activated cell sorter (FACS) analysis of HEK 293T cells (blue outline) incubated with carboxyfluorescein succinimidyl ester (CFSE)-labeled wild-type ATCC 23726 in the presence of
d
-galactose (gal) or with labeled hemagglutinin-deficient mutants (red outline) D22, K25, and K50 and J78, a randomly selected mutant used as control. Wild-type ATCC 23726 control is represented by the gray-filled histogram. Mean fluorescence intensity values are indicated for each histogram. Values for the wild type are in black, and those for the galactose-treated bacteria and the mutants are in red. Data are representative of three independent experiments.
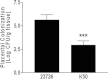
FIG 6
The Fap2 adhesin is involved in placental colonization. Wild-type F. nucleatum ATCC 23726 or the hemagglutination-deficient mutant K50 was injected into the tail veins of pregnant mice. After 24 h, the placentas were harvested and homogenized, and bacterial CFU were determined. ***, P < 0.001.
Similar articles
- Fluorescence based measurements of Fusobacterium nucleatum coaggregation and of fusobacterial attachment to mammalian cells.
Bachrach G, Ianculovici C, Naor R, Weiss EI. Bachrach G, et al. FEMS Microbiol Lett. 2005 Jul 15;248(2):235-40. doi: 10.1016/j.femsle.2005.05.055. FEMS Microbiol Lett. 2005. PMID: 15993010 - The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm.
Kaplan CW, Lux R, Haake SK, Shi W. Kaplan CW, et al. Mol Microbiol. 2009 Jan;71(1):35-47. doi: 10.1111/j.1365-2958.2008.06503.x. Epub 2008 Nov 7. Mol Microbiol. 2009. PMID: 19007407 Free PMC article. - Complementation of the fadA mutation in Fusobacterium nucleatum demonstrates that the surface-exposed adhesin promotes cellular invasion and placental colonization.
Ikegami A, Chung P, Han YW. Ikegami A, et al. Infect Immun. 2009 Jul;77(7):3075-9. doi: 10.1128/IAI.00209-09. Epub 2009 Apr 27. Infect Immun. 2009. PMID: 19398541 Free PMC article. - Fusobacterium nucleatum: a commensal-turned pathogen.
Han YW. Han YW. Curr Opin Microbiol. 2015 Feb;23:141-7. doi: 10.1016/j.mib.2014.11.013. Epub 2015 Jan 8. Curr Opin Microbiol. 2015. PMID: 25576662 Free PMC article. Review. - The ways Fusobacterium nucleatum translocate to breast tissue and contribute to breast cancer development.
Guo X, Yu K, Huang R. Guo X, et al. Mol Oral Microbiol. 2024 Feb;39(1):1-11. doi: 10.1111/omi.12446. Mol Oral Microbiol. 2024. PMID: 38171827 Review.
Cited by
- Spatial scale in analysis of the dental plaque microbiome.
Borisy GG, Valm AM. Borisy GG, et al. Periodontol 2000. 2021 Jun;86(1):97-112. doi: 10.1111/prd.12364. Epub 2021 Mar 10. Periodontol 2000. 2021. PMID: 33690940 Free PMC article. Review. - Alterations in the gastric microbiota and metabolites in gastric cancer: An update review.
Lei C, Gong D, Zhuang B, Zhang Z. Lei C, et al. Front Oncol. 2022 Aug 23;12:960281. doi: 10.3389/fonc.2022.960281. eCollection 2022. Front Oncol. 2022. PMID: 36081564 Free PMC article. Review. - Fusobacterium nucleatum induces invasive growth and angiogenic responses in malignant oral keratinocytes that are cell line- and bacterial strain-specific.
Selvaraj A, McManus G, Healy CM, Moran GP. Selvaraj A, et al. Front Cell Infect Microbiol. 2024 Sep 2;14:1417946. doi: 10.3389/fcimb.2024.1417946. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39286811 Free PMC article. - Growth Conditions Influence Lactobacillus Cell-Free Supernatant Impact on Viability, Biofilm Formation, and Co-Aggregation of the Oral Periodontopathogens Fusobacterium nucleatum and Porphyromonas gingivalis.
Zanetta P, Squarzanti DF, di Coste A, Amoruso A, Pane M, Azzimonti B. Zanetta P, et al. Biomedicines. 2023 Mar 11;11(3):859. doi: 10.3390/biomedicines11030859. Biomedicines. 2023. PMID: 36979838 Free PMC article. - Microbial dysbiosis in oral squamous cell carcinoma: A systematic review and meta-analysis.
Yu X, Shi Y, Yuan R, Chen Z, Dong Q, Han L, Wang L, Zhou J. Yu X, et al. Heliyon. 2023 Jan 24;9(2):e13198. doi: 10.1016/j.heliyon.2023.e13198. eCollection 2023 Feb. Heliyon. 2023. PMID: 36793959 Free PMC article. Review.
References
- Swidsinski A, Dorffel Y, Loening-Baucke V, Theissig F, Ruckert JC, Ismail M, Rau WA, Gaschler D, Weizenegger M, Kuhn S, Schilling J, Dorffel WV. 2011. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 60:34–40. doi:10.1136/gut.2009.191320. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources