IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production - PubMed (original) (raw)
doi: 10.1038/mi.2014.123. Epub 2015 Jan 7.
T Wen 2, M K Mingler 2, J M Caldwell 2, Y H Wang 2, D D Chaplin 3, E H Lee 4, M H Jang 5, S Y Woo 6, J Y Seoh 6, M Miyasaka 7, M E Rothenberg 2
Affiliations
- PMID: 25563499
- PMCID: PMC4481137
- DOI: 10.1038/mi.2014.123
IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production
Y Jung et al. Mucosal Immunol. 2015 Jul.
Abstract
Eosinophils are multifunctional leukocytes that reside in the gastrointestinal (GI) lamina propria, where their basal function remains largely unexplored. In this study, by examining mice with a selective deficiency of systemic eosinophils (by lineage ablation) or GI eosinophils (eotaxin-1/2 double deficient or CC chemokine receptor 3 deficient), we show that eosinophils support immunoglobulin A (IgA) class switching, maintain intestinal mucus secretions, affect intestinal microbial composition, and promote the development of Peyer's patches. Eosinophil-deficient mice showed reduced expression of mediators of secretory IgA production, including intestinal interleukin 1β (IL-1β), inducible nitric oxide synthase, lymphotoxin (LT) α, and LT-β, and reduced levels of retinoic acid-related orphan receptor gamma t-positive (ROR-γt(+)) innate lymphoid cells (ILCs), while maintaining normal levels of APRIL (a proliferation-inducing ligand), BAFF (B cell-activating factor of the tumor necrosis factor family), and TGF-β (transforming growth factor β). GI eosinophils expressed a relatively high level of IL-1β, and IL-1β-deficient mice manifested the altered gene expression profiles observed in eosinophil-deficient mice and decreased levels of IgA(+) cells and ROR-γt(+) ILCs. On the basis of these collective data, we propose that eosinophils are required for homeostatic intestinal immune responses including IgA production and that their affect is mediated via IL-1β in the small intestine.
Figures
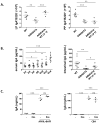
Figure 1. Reduced immunoglobulin A (IgA) levels in serum and intestinal lavage of eosinophil-deficient mice and their decreased IgA+ cell numbers in the small intestine and Peyer’s patches (PP)
(A) The small intestinal lamina propria (LP) cells of the wild-type (WT; black circles), ΔdblGATA, CC chemokine receptor 3–deficient (_Ccr_3 knockout [KO]), eotaxin-1/2 double–deficient (Ccl11/Ccl24 double knock out [DKO]), and PHIL mice (eosinophil deficient; white circles). The frequencies of CD11bhighCD11cint small intestinal eosinophils were analyzed with R1-gated cells representing the medium-to-high SSC subset. (B) IgA levels in intestinal lavage and serum of WT and eosinophil-deficient mice (ΔdblGATA, Ccr3 KO, Ccl11/Ccl24 DKO, and PHIL). (C) Frequencies and/or numbers of IgA+ cells in the small intestinal LP, PP, and mesenteric lymph nodes (MLN) of WT, ΔdblGATA, Ccr3 KO, Ccl11/Ccl24 DKO, and PHIL mice. All data are representative of two or more independent experiments. Data are mean ± SEM values. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s _t_-test).
Figure 2. Small intestine and Peyer’s patches (PP) immune cell populations in eosinophil-deficient mice and impaired PP development
(A) Total lamina propria (LP) cells and mononuclear cell (MNC) numbers in the small intestine of wild-type (WT; black circles), ΔdblGATA, CC chemokine receptor 3–deficient (_Ccr_3 knockout [KO]), eotaxin-1/2 double–deficient (Ccl11/Ccl24 double knock out [DKO]), and PHIL mice (eosinophil deficient; white circles). (B) Weight and cell numbers of PP isolated from WT and eosinophil-deficient mice. (C) Hematoxylin and eosin (H&E) staining of PP in WT and eosinophil-deficient mice. Arrowheads indicate PP in the small intestine. Original magnification × 10. All data are representative of two or more independent experiments with n ≥ 3 mice. Data are mean ± standard error of the mean (SEM) values. *P < 0.05, **P < 0.01 (Student’s _t_-test).
Figure 3. Effect of adoptively transferred small intestinal lamina propria (LP) cells on immunoglobulin A (IgA) synthesis in ΔdblGATA mice
(A) Numbers of IgA+ cells in the LP of small intestine and Peyer’s patches (PP) of wild-type mice (WT; black circles), ΔdblGATA (eosinophil-deficient; white circles), and ΔdblGATA mice after adoptive transfer of small intestinal LP cells isolated from WT mice (ΔdblGATA WT LP Adoptive Tf; white circles). Data are mean ± standard error of the mean (SEM) values. **P < 0.01, ***P < 0.001 (Student’s _t_-test). (B) IgA levels in serum of ΔdblGATA mice were analyzed over the indicated time period after adoptive transfer of WT LP cells. IgA levels in intestinal lavage of WT, ΔdblGATA mice, and ΔdblGATA mice 14 days after adoptive transfer of WT LP cells. Data are mean ± standard error of the mean (SEM) values. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s _t_-test). (C) Naïve B cells isolated from the small intestine of WT mice were cultured with the indicated stimuli in the presence or absence of small intestinal eosinophils for 7 days. IgA production by stimulated B cells was determined by ELISA. All data are representative of two or more independent experiments. Data are mean ± standard error of the mean (SEM) values. ***P < 0.001 (Student’s _t_-test).
Figure 4. Decreased mucus secretion and less efficient induction of oral tolerance in ΔdblGATA mice
(A) The mucus layer of wild-type (WT) and ΔdblGATA were visualized by immunofluorescence staining of small intestine with wheat germ agglutinin (WGA; green) and phalloidin (red). Sections were ounterstained with DAPI (blue). Original magnification × 40. The WGA-positive and phalloidin-positive luminal areas were measured using Imaris software. Three sections were examined in each group (WT, black circles; ΔdblGATA, white circles) and 5 to 6 fields were selected from 1 section. Data are mean ± standard error of the mean (SEM) values. ***P < 0.001 (Student’s _t_-test). (B) Ovalbumin (OVA)-specific serum immunoglobulin (Ig) G1, IgG2a, and IgE titers were compared between OVA-fed, sensitized mice (Tol) and sensitized mice without OVA (IP) in WT and ΔdblGATA mice. The data are representative of more than three independent experiments. Data are mean ± standard error of the mean (SEM) values. *P < 0.05, **P < 0.01 (Student’s _t_-test).
Figure 5. Expression of lymphotoxin α (Lta) and β (Ltb) are decreased in the small intestine of ΔdblGATA mice
(A) Levels of a proliferation-inducing ligand (Tnfsf13), B cell–activating factor of the tumor necrosis factor (Tnfsf13b), and transforming growth factor β (Tgfb1) mRNA in the small intestine (SI) and Peyer’s patches (PP) of wild-type (WT; black circles) and ΔdblGATA (white circles) mice. (B) Eosinophils isolated from the small intestine of WT mice were subjected to genome-wide mRNA microarray using Affymetrix mouse ST 1.0 chip. The raw expression values of Tnfsf13, Tnfsf13b, and Tgfb1 were displayed, with a threshold value of 400 units regarded as significant expression. (C) Levels of Lta and Ltb mRNA in the small intestine and PP of WT and ΔdblGATA mice. (D) The Affymetrix raw expression value of Lta and Ltb in eosinophils isolated from the small intestine of WT mice. All data, except for microarray analysis, are representative of two or more independent experiments. Data are mean ± standard error of the mean (SEM) values. *P < 0.05, **P < 0.01 (Student’s _t_-test).
Figure 6. Small intestinal eosinophils actively express IL-1β
(A) The Affymetrix raw expression value of interleukin (IL) 1β (Il1b), IL-23 (Il23a), IL-25 (Il25), IL-33 (Il33), IL-2 (Il2), and IL-7 (Il7) in eosinophils isolated from small intestinal lamina propria of wild-type (WT) mice, with a threshold value of 400 units regarded as significant expression. (B) Il1b mRNA levels in the small intestine (SI) and Peyer’s patches (PP) of WT (black circles) and ΔdblGATA (white circles) mice. (C) IL-1β production was measured from cultures of small intestinal eosinophils (Eos) or non-eosinophils (NEos, SSClowCD45+MHC II+CD11b−CD11c−) subsets with the indicated stimuli for 24 hours. (D) IL-1β was detected with the indicated small intestinal segments of WT and ΔdblGATA mice. 1 mg of protein extracted from each of the segment was loaded for ELISA. All data, except for microarray analysis, are representative of two or more independent experiments. Data are mean ± standard error of the mean (SEM) values. **P < 0.01, ***P < 0.001 (Student’s _t_-test).
Figure 7. The level of retinoic acid-related orphan receptor gamma t–positive (ROR- γt+) innate lymphoid cells (ILCs) and inducible nitric oxide synthase (iNOS, Nos2) expression in interleukin (IL) 1β–deficient (Il1b knock out [KO]) and ΔdblGATA mice
(A) Numbers of small intestinal lamina propria (LP) IgA+ cells and intestinal IgA levels in wild-type (WT; black circles) and Il1b KO (black squares) mice. (B) ROR- γt+ ILC numbers and expression of lymphotoxin α (Lta) and β (Ltb) mRNA in the small intestine of WT and Il1b KO mice. Lineage marker (Lin) was the combination of CD3, B220, CD11c, and Gr-1. (C) Nos2 mRNA expressions in the small intestine of WT and Il1b KO mice. (D) ROR- γt+ ILC numbers in the small intestine of WT and ΔdblGATA (white circles) mice. (E) Nos2 mRNA expression in the small intestine (SI) and PP of WT and ΔdblGATA mice. All data are representative of two or more independent experiments. Data are mean ± standard error of the mean (SEM) values. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s _t_-test).
Figure 8. Changes in the microbiota of ΔdblGATA mice
(A) Sequencing analysis of microbiota composition from the stool of wild-type (WT) and ΔdblGATA mice. Representative microbiota composition is presented (n = 9 mice per group). (B) Real-time polymerase chain reaction of microbiota in the stool of WT and ΔdblGATA mice. (C) Interleukin 7 (Il7) mRNA expression in the small intestine of WT (black circles) and ΔdblGATA (white circles) mice. All data, except for sequencing analysis, are representative of two or more independent experiments. Data are mean ± standard error of the mean (SEM) values. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s _t_-test).
Similar articles
- Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract.
Yamamoto M, Rennert P, McGhee JR, Kweon MN, Yamamoto S, Dohi T, Otake S, Bluethmann H, Fujihashi K, Kiyono H. Yamamoto M, et al. J Immunol. 2000 May 15;164(10):5184-91. doi: 10.4049/jimmunol.164.10.5184. J Immunol. 2000. PMID: 10799877 - Re-thinking the functions of IgA(+) plasma cells.
Gommerman JL, Rojas OL, Fritz JH. Gommerman JL, et al. Gut Microbes. 2014;5(5):652-62. doi: 10.4161/19490976.2014.969977. Gut Microbes. 2014. PMID: 25483334 Free PMC article. Review. - Specific microbiota enhances intestinal IgA levels by inducing TGF-β in T follicular helper cells of Peyer's patches in mice.
Beller A, Kruglov A, Durek P, von Goetze V, Werner K, Heinz GA, Ninnemann J, Lehmann K, Maier R, Hoffmann U, Riedel R, Heiking K, Zimmermann J, Siegmund B, Mashreghi MF, Radbruch A, Chang HD. Beller A, et al. Eur J Immunol. 2020 Jun;50(6):783-794. doi: 10.1002/eji.201948474. Epub 2020 Feb 27. Eur J Immunol. 2020. PMID: 32065660 - Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis.
Chu VT, Beller A, Rausch S, Strandmark J, Zänker M, Arbach O, Kruglov A, Berek C. Chu VT, et al. Immunity. 2014 Apr 17;40(4):582-93. doi: 10.1016/j.immuni.2014.02.014. Immunity. 2014. PMID: 24745334 - Gastrointestinal eosinophils.
Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Rothenberg ME, et al. Immunol Rev. 2001 Feb;179:139-55. doi: 10.1034/j.1600-065x.2001.790114.x. Immunol Rev. 2001. PMID: 11292017 Review.
Cited by
- Active eosinophils regulate host defence and immune responses in colitis.
Gurtner A, Borrelli C, Gonzalez-Perez I, Bach K, Acar IE, Núñez NG, Crepaz D, Handler K, Vu VP, Lafzi A, Stirm K, Raju D, Gschwend J, Basler K, Schneider C, Slack E, Valenta T, Becher B, Krebs P, Moor AE, Arnold IC. Gurtner A, et al. Nature. 2023 Mar;615(7950):151-157. doi: 10.1038/s41586-022-05628-7. Epub 2022 Dec 12. Nature. 2023. PMID: 36509106 Free PMC article. - Living without eosinophils: evidence from mouse and man.
Jackson DJ, Pavord ID. Jackson DJ, et al. Eur Respir J. 2023 Jan 12;61(1):2201217. doi: 10.1183/13993003.01217-2022. Print 2023 Jan. Eur Respir J. 2023. PMID: 35953100 Free PMC article. Review. - Eosinophil Activation by Toll-Like Receptor 4 Ligands Regulates Macrophage Polarization.
Yoon J, Um HN, Jang J, Bae YA, Park WJ, Kim HJ, Yoon MS, Chung IY, Jung Y. Yoon J, et al. Front Cell Dev Biol. 2019 Dec 20;7:329. doi: 10.3389/fcell.2019.00329. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31921842 Free PMC article. - Small intestinal eosinophils regulate Th17 cells by producing IL-1 receptor antagonist.
Sugawara R, Lee EJ, Jang MS, Jeun EJ, Hong CP, Kim JH, Park A, Yun CH, Hong SW, Kim YM, Seoh JY, Jung Y, Surh CD, Miyasaka M, Yang BG, Jang MH. Sugawara R, et al. J Exp Med. 2016 Apr 4;213(4):555-67. doi: 10.1084/jem.20141388. Epub 2016 Mar 7. J Exp Med. 2016. PMID: 26951334 Free PMC article. - Regulatory Eosinophils in Inflammation and Metabolic Disorders.
Yang BG, Seoh JY, Jang MH. Yang BG, et al. Immune Netw. 2017 Feb;17(1):41-47. doi: 10.4110/in.2017.17.1.41. Epub 2017 Feb 23. Immune Netw. 2017. PMID: 28261019 Free PMC article. Review.
References
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. - PubMed
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK078392/DK/NIDDK NIH HHS/United States
- R01 AI045898/AI/NIAID NIH HHS/United States
- R01 AI083450/AI/NIAID NIH HHS/United States
- R37 AI045898/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous