Structural insight into operator dre-sites recognition and effector binding in the GntR/HutC transcription regulator NagR - PubMed (original) (raw)
Structural insight into operator dre-sites recognition and effector binding in the GntR/HutC transcription regulator NagR
Simon B Fillenberg et al. Nucleic Acids Res. 2015 Jan.
Abstract
The uptake and metabolism of N-acetylglucosamine (GlcNAc) in Bacillus subtilis is controlled by NagR (formerly named YvoA), a member of the widely-occurring GntR/HutC family of transcription regulators. Upon binding to specific DNA operator sites (dre-sites) NagR blocks the transcription of genes for GlcNAc utilization and interaction of NagR with effectors abrogates gene repression. Here we report crystal structures of NagR in complex with operator DNA and in complex with the putative effector molecules glucosamine-6-phosphate (GlcN-6-P) and N-acetylglucosamine-6-phosphate (GlcNAc-6-P). A comparison of the distinct conformational states suggests that effectors are able to displace the NagR-DNA-binding domains (NagR-DBDs) by almost 70 Å upon binding. In addition, a high-resolution crystal structure of isolated NagR-DBDs in complex with palindromic double-stranded DNA (dsDNA) discloses both the determinants for highly sequence-specific operator dre-site recognition and for the unspecific binding of NagR to dsDNA. Extensive biochemical binding studies investigating the affinities of full-length NagR and isolated NagR-DBDs for either random DNA, dre-site-derived palindromic or naturally occurring non-palindromic dre-site sequences suggest that proper NagR function relies on an effector-induced fine-tuning of the DNA-binding affinities of NagR and not on a complete abrogation of its DNA binding.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
Figure 1.
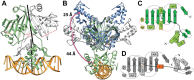
Crystal structure and topology plot of ligand-bound NagR. (A) Dimer of NagR in complex with GlcNAc-6-P in a cartoon representation with the monomers colored in blue and light gray and the ligand GlcNAc-6-P shown as a stick-model. (B) Side view of the complex after a 90° rotation. (C) Topology plot of monomeric NagR in complex with GlcNAc-6-P. Secondary structure elements are displayed as light blue cylinders (α-helices) and blue arrows (β-strands). The linker region between the DNA- and the effector-binding domain is highlighted in bold. The newly formed β-strand β* that appears upon ligand binding is colored in orange in all panels.
Figure 2.
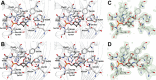
Closeup view of the effector-binding site of NagR. (A) and (B) Stereo view showing the interactions of (A) GlcN-6-P and (B) GlcNAc-6-P with NagR. Ligands and residues are presented as stick models and water molecules as red spheres. (C) and (D) Simulated annealing _F_o_-F_c omit maps showing (C) the GlcN-6-P and (D) GlcNAc-6-P-binding site of NagR in the respective structures. Maps were calculated with PHENIX and are contoured as green mesh at 3.0 σ. Ligand molecules and all residues omitted during refinement and map calculation are shown. Chain B of the two NagR chains present in each crystal structure was selected arbitrarily for generating all the illustrations.
Figure 3.
Crystal structure of wild-type NagR in complex with 19mer palindromic dsDNA. (A) Dimer of NagR in complex with palindromic dsDNA in a cartoon representation with the monomers colored in green and light gray. Segments with missing electron density in the structure of NagR are highlighted as red springs. The non-crystallographic symmetry axes relating the two protein chains in the DBD dimer as well as in the effector-binding domain dimer were generated with Chimera and are shown as black rods. (B) Superposition of the crystal structures of NagR in complex with palindromic dsDNA (green, PDB ID: 4WWC), sulfate-bound NagR (light gray, PDB ID: 2WV0) and GlcNAc-6-P-bound NagR (blue, PDB ID: 4U0W) in a cartoon representation. Sulfate molecules present in 2WV0 are omitted for clarity. The center of mass for one DBD of each dimer was calculated with Chimera and is presented as a pink sphere. (C) Topology plot of monomeric NagR in its DNA-bound conformation. Secondary structure elements and loop regions that are missing in chain A and/or B are marked with a red dotted line. (D) Topology plot of monomeric NagR in complex with a bound sulfate molecule (7). Helix α* that is only present in the sulfate-bound NagR structure is colored in orange. In panels (C) and (D) the secondary structure elements are displayed as cylinders (α helices) and arrows (β-strands). The linker region between the DNA- and the effector-binding domain is highlighted by a bold line.
Figure 4.
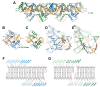
Crystal structure of NagR–DBD in complex with 15mer palindromic dsDNA. (A) The crystallographic asymmetric unit (ASU) comprises four NagR–DBD monomers bound to dsDNA (middle; delineated by red dotted lines). The complex is shown together with two adjacent ASUs that are related by a crystallographic 31 screw axis as indicated. All molecules are shown in a cartoon representation with the centrally-bound NagR–DBD dimer colored in blue and light blue. The edge-bound NagR–DBD dimer is depicted in green and light green. (B) and (C) Close-up view of (B) a centrally-bound NagR–DBD dimer bound to dsDNA forming the _dre_-site-specific recognition complex and of (C) an edge-bridging NagR–DBD dimer that reveals non-_dre_-site-specific binding interactions. (D) and (E) Details of the interactions between DNA and (D) a centrally-bound NagR–DBD and (E) an edge-bound NagR–DBD. Only base-directed interactions are shown. Hydrogen bonds are represented by red dotted lines. Interacting residues and bases are shown as stick models. (F) and (G) Schematic summary of the NagR–DBD–DNA contacts formed by (F) the centrally-bound dimer and (G) the edge-bound dimer. Only direct interactions, identified with the analysis software NUCPLOT (30), are shown. Base-specific contacts are indicated in red. Nucleotides in the recognition half-sites are numbered according to their position from the center of the palindrome.
Figure 5.
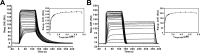
Quantitative analysis of NagR–DBD interactions with _dre_-site-containing dsDNA constructs. Sensorgrams from SPR analyses of the interaction of NagR–DBD with (A) palindromic and (B) native nagAB dsDNA for the respective triplicate measurements are shown. The corresponding diagrams for the determination of _K_D values are also included and display the SPR response units plotted versus the NagR–DBD concentrations which were fitted to the Langmuir equation for a 2:1 and a 1:1 binding reaction, respectively. The error bars indicate the standard deviation among triplicate data. The concentrations of NagR–DBD ranged from 20 nM to 7.5 μM for palindromic dsDNA and from 25 nM to 7.5 μM for native nagAB dsDNA.
Figure 6.
Quantitative analysis of NagR interactions with _dre_-site-containing dsDNA constructs. Sensorgrams from SPR analyses of the interaction of NagR with (A) palindromic and (B) native nagAB dsDNA of the respective triplicate measurements are shown. Kinetic analysis showed best fit to a 1:1 Langmuir type of interaction. From this interaction model the association rate constants (_k_a, corresponding fits shown as black curves) and dissociation rate constants (_k_d) were determined with values of _k_a = 1.4 ± 0.3 × 107 M−1s−1, _k_d = 2.3 ± 0.4 × 10−4 s−1 and _k_a = 1.8 ± 0.7 × 107 M−1s−1, _k_d = 1.7 ± 0.4 × 10−4 s−1 for the interaction with palindromic and native nagAB dsDNA, respectively. These values were used to calculate the equilibrium dissociation constants (_K_D) reported in Table 2. The NagR concentrations used are listed on the right side of the respective sensorgram.
Figure 7.
Analytical size exclusion chromatography runs of (A) NagR and (B) NagR–DBD showing the interaction with various 15mer dsDNA constructs derived from _Bacillus subtilis dre_-sites. The final concentrations of dimeric NagR and monomeric NagR–DBD are indicated for each curve. The dsDNA constructs were used with a final concentration of 3 μM.
Figure 8.
Quantitative analysis of the interaction of NagR with non-_dre_-site control dsDNA fragments. Sensorgrams from SPR analyses of the interaction of NagR with (A) a nagA gene derived and (B) a randomly designed control dsDNA of the respective triplicate measurements are shown. The corresponding diagrams for the determination of _K_D values are also included and display the SPR response units plotted versus the NagR concentrations. A Langmuir 1:1 binding model was applied. The error bars indicate standard deviations between triplicate data. The corresponding NagR concentrations ranged from 30 nM to 2 μM for both dsDNA constructs.
Figure 9.
Sequence conservation in NagR transcription factor-binding sites in Bacillales. The nucleotide preference was calculated from the binding site sequences listed in Supplementary Table S2 and was generated with WebLogo 3.3 (34). GC bases are colored in gray, AT bases in blue. Nucleotides anticipated to participate in sequence-specific transcription factor binding are marked in red. Gly69 (wing motif), Arg38 (αD2) and Arg48 (αD3) are the NagR residues involved in base-specific DNA binding. These residues bind either to the listed segment or to the complementary strand.
Figure 10.
Effector binding and concomitant structural rearrangements modulate DNA binding in NagR. Graphical summary of the DNA-binding modes and affinities observed for NagR. Dimer-forming monomers of NagR are illustrated schematically in blue. Effector molecules are depicted as red hexagons. _Dre_-site-specific and unspecific dsDNA is colored in orange and gray, respectively.
Similar articles
- Crystal Structures of the Global Regulator DasR from Streptomyces coelicolor: Implications for the Allosteric Regulation of GntR/HutC Repressors.
Fillenberg SB, Friess MD, Körner S, Böckmann RA, Muller YA. Fillenberg SB, et al. PLoS One. 2016 Jun 23;11(6):e0157691. doi: 10.1371/journal.pone.0157691. eCollection 2016. PLoS One. 2016. PMID: 27337024 Free PMC article. - Regulation of amino sugar utilization in Bacillus subtilis by the GntR family regulators, NagR and GamR.
Gaugué I, Oberto J, Plumbridge J. Gaugué I, et al. Mol Microbiol. 2014 Apr;92(1):100-15. doi: 10.1111/mmi.12544. Epub 2014 Mar 5. Mol Microbiol. 2014. PMID: 24673833 - NagR Differentially Regulates the Expression of the glmS and nagAB Genes Required for Amino Sugar Metabolism by Streptococcus mutans.
Zeng L, Burne RA. Zeng L, et al. J Bacteriol. 2015 Nov;197(22):3533-44. doi: 10.1128/JB.00606-15. Epub 2015 Aug 31. J Bacteriol. 2015. PMID: 26324448 Free PMC article. - Regulon of the N-acetylglucosamine utilization regulator NagR in Bacillus subtilis.
Bertram R, Rigali S, Wood N, Lulko AT, Kuipers OP, Titgemeyer F. Bertram R, et al. J Bacteriol. 2011 Jul;193(14):3525-36. doi: 10.1128/JB.00264-11. Epub 2011 May 20. J Bacteriol. 2011. PMID: 21602348 Free PMC article. - Allosteric control of transcription in GntR family of transcription regulators: A structural overview.
Jain D. Jain D. IUBMB Life. 2015 Jul;67(7):556-63. doi: 10.1002/iub.1401. Epub 2015 Jul 14. IUBMB Life. 2015. PMID: 26172911 Review.
Cited by
- Structural and Functional Characterization of Rv0792c from Mycobacterium tuberculosis: Identifying Small Molecule Inhibitor against HutC Protein.
Chauhan NK, Anand A, Sharma A, Dhiman K, Gosain TP, Singh P, Singh P, Khan E, Chattopadhyay G, Kumar A, Sharma D, Ashish, Sharma TK, Singh R. Chauhan NK, et al. Microbiol Spectr. 2023 Feb 14;11(1):e0197322. doi: 10.1128/spectrum.01973-22. Epub 2022 Dec 12. Microbiol Spectr. 2023. PMID: 36507689 Free PMC article. - NupR Responding to Multiple Signals Is a Nucleoside Permease Regulator in Bacillus thuringiensis BMB171.
Qin J, Cao Z, Cai X, Fang Y, An B, Li X, Zhang Y, Tian H, Hu W, Yan B, Cai J. Qin J, et al. Microbiol Spectr. 2022 Aug 31;10(4):e0154322. doi: 10.1128/spectrum.01543-22. Epub 2022 Jul 7. Microbiol Spectr. 2022. PMID: 35862946 Free PMC article. - Characterization of a Salmonella Transcription Factor-DNA Complex and Identification of the Inducer by Native Mass Spectrometry.
Szkoda BE, Di Capua A, Shaffer J, Behrman EJ, Wysocki VH, Gopalan V. Szkoda BE, et al. J Mol Biol. 2022 Apr 15;434(7):167480. doi: 10.1016/j.jmb.2022.167480. Epub 2022 Feb 14. J Mol Biol. 2022. PMID: 35176290 Free PMC article. - Mechanism of NanR gene repression and allosteric induction of bacterial sialic acid metabolism.
Horne CR, Venugopal H, Panjikar S, Wood DM, Henrickson A, Brookes E, North RA, Murphy JM, Friemann R, Griffin MDW, Ramm G, Demeler B, Dobson RCJ. Horne CR, et al. Nat Commun. 2021 Mar 31;12(1):1988. doi: 10.1038/s41467-021-22253-6. Nat Commun. 2021. PMID: 33790291 Free PMC article.
References
- Stülke J., Hillen W. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 2000;54:849–880. - PubMed
- Gorke B., Stulke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008;6:613–624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases