Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner - PubMed (original) (raw)
Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner
Vladimir G Gainullin et al. J Clin Invest. 2015 Feb.
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a common inherited nephropathy responsible for 4%-10% of end-stage renal disease cases. Mutations in the genes encoding polycystin-1 (PC1, PKD1) or polycystin-2 (PC2, PKD2) cause ADPKD, and PKD1 mutations are associated with more severe renal disease. PC1 has been shown to form a complex with PC2, and the severity of PKD1-mediated disease is associated with the level of the mature PC1 glycoform. Here, we demonstrated that PC1 and PC2 first interact in the ER before PC1 cleavage at the GPS/GAIN site and determined that PC2 acts as an essential chaperone for PC1 maturation and surface localization. The chaperone function of PC2 was dependent on the presence of the distal coiled-coil domain and was disrupted by pathogenic missense mutations. In Pkd2-/- mice, complete loss of PC2 prevented PC1 maturation. In Pkd2 heterozygotes, the 50% PC2 reduction resulted in a nonequimolar reduction (20%-25%) of the mature PC1 glycoform. Interbreeding between various Pkd1 and Pkd2 models revealed that animals with reduced levels of functional PC1 and PC2 in the kidney exhibited severe, rapidly progressive disease, illustrating the importance of complexing of these proteins for function. Our results indicate that PC2 regulates PC1 maturation; therefore, mature PC1 levels are a determinant of disease severity in PKD2 as well as PKD1.
Figures
Figure 8. Deletion of CC2 and missense mutations in PC2 influence PC1 maturation.
(A) Diagram of WT and mutant human PC2 used in the cotransfection experiments (B and C) showing the positions of domains, deletions, and aa substitutions. (B) mCherry-PC1 cotransfected with WT GFP-PC2 or PC2 lacking the C-terminal cytoplasmic tail (GFP-PC2-L703X) into RCTE cells. Detection of PC1 NT (mCherry) revealed EndoH-resistant PC1-NTR (arrow) only in WT-PC2, but not in PC2-L703X, cotransfected cells. Detection of PC2 (GFP) shows that a portion of PC2-L703X was EndoH resistant (R; arrow). Representative blots are shown from 3 independent experiments. (C) Cotransfection of various GFP-PC2 mutants and mCherry-PC1 into RCTE cells showing the effect of PC2 mutations on the PC1 glycosylation pattern and PC2 products. Deletion of the EF hand and coiled coil 1 (delEF+CC1) did not disrupt PC1 maturation, but deletion of coiled coil 2 (delCC2) greatly reduced the level of the PC1-NTR product. Pathogenic missense substitutions in PC2, especially p.R322Q and p.W414G, also had a negative effect on PC1 maturation. Representative blots are shown from 3 independent experiments. (D) Quantification of the ratio of PC1-NTR to NTS glycoforms obtained from the cotransfection experiments with WT and mutant PC2 GFP constructs (C and Supplemental Figure 5A). Deletion mutants removing CC2 but not EF+CC1 largely disrupted PC1 maturation, while missense mutations also significantly disrupted maturation. n = 3 for all except R322W (n = 2) and D511V (n = 4). Quartile box plots represent the median, quartiles, and minimum/maximum range, with the mean of each group in parentheses. P_values are shown as compared with GFP-PC2-WT (control) with the Student’s_t test; ****P < 0.0001.
Figure 7. Analysis of maturation of endogenous PC1 truncation mutants.
(A) Diagram showing approximate locations of the truncations in PC1 and the locations of the REJ domain, PKD repeats, and CT-CC. (B) Glycosylation analysis of MEFs isolated from WT and Pkd1del31/del31_embryos detected with the PC1 NT antibody. The del31 mutation truncated PC1 after the GPS/GAIN cleavage site, and since the truncated protein was cleaved, an NT as well as a truncated FL (tFL) product were observed. EndoH analysis shows that PC1-NTS, but not PC1-NTR, was generated. Representative blots are shown from 3 independent experiments. (C) IP of endogenous PC2 (H280) in WT and_Pkd1del31/del31 (–/–) MEFs shows that PC2 did not coimmunoprecipitate with the del31 mutant PC1. Lysate control is shown above. Representative blots are shown from 3 independent experiments. (D–G) Pkd1del17/+ (WT/del17) adult mouse kidney (D and E) and human fibroblasts from a female ADPKD patient with an extracellular truncation due to a translocation in exon 15 (WT/tr15; 77-2) (F and G), disrupting PC1 extracellularly in the REJ domain or PKD1 repeats, respectively. (D, F, andG) Glycosylation analysis comparing untreated (Un), EndoH-digested (+E), and PNGase F–digested (+P) protein. In all cases, only the EndoH-sensitive truncated product (PC1-tNTS) was seen, with no PC1-tNTR glycoform. In del17 and tr15, the PC1 truncated product was expressed at a much higher level than that detected WT, and so longer exposures are shown (E and G) to visualize the WT allele/products. Representative blots are shown from 3 independent experiments.
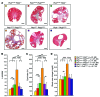
Figure 6. Pkd2 depletion aggravates the Pkd1RC/RC cystic phenotype.
(A) Masson trichrome–stained kidney cross sections of 4-month-old mice of the Pkd1RC/RC genotype with the addition of_Pkd2WS25/+, Pkd2+/–, or_Pkd2WS25/–, and Pkd2WS25/–_mice with the Pkd1RC/+ genotype. PKD severity and fibrosis worsened in bigenic mice, with evidently more severe disease in the_Pkd1RC/RC Pkd2+/– genotype, corresponding to PC1-NTR levels of about 30% (Figure 5C). However, the Pkd1RC/RC Pkd2WS25/– combination resulted in the most severe disease. Scale bar: 1 mm. (B–D) Graphical representations of %KW/BW (B), cystic index (C), and blood urea nitrogen (D) of the various genotypes quantify the increased disease severity with Pkd1/Pkd2 combined phenotypes (see Supplemental Table 1 for details). Error bars depict ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using a 2-way ANOVA with Student’s t test. F, female; M, male. +Note that 5 of 10 Pkd1RC/RC Pkd2WS25/– animals died before 4 months (F: P42, P74; M: P38, P51, P79).
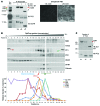
Figure 5. Maturation of PC1 is associated with the dosage of PC2.
(A) Membrane protein purified from P9 mouse kidneys of WT,Pkd2+/–, Pkd1+/–, and bigenic combinations with the Pkd1RC/RC genotype and_Pkd2WS25_ allele assayed by SDS-PAGE and probed with PC1 NT and PC2 antibodies. Densitometric profiles of the NT products are shown with a Coomassie-stained loading control. Reduction of Pkd2 reduced the level of PC1-NTR, while Pkd1 reduction lowered the level of both products. Note in Pkd1RC/RC animals that the PC1-FL product is more evident, consistent with the previously described partial cleavage defect (20). Representative blots are shown from 3 independent experiments. (B) IB of membrane-purified protein from WT, Pkd1RC/RCPkd2+/+, and Pkd1RC/RCPkd2+/– MEFs detected with PC1 NT or PC2 antibodies showing the PC1-FL, PC1-NTR, and PC1-NTS glycoforms, PC2, and control Coomassie band. Representative blots are shown from 3 independent experiments. (C) Quantification of PC1-NTR from MEFs with various Pkd1 and _Pkd2_genotypes. Results were derived from a minimum of 3 independent IBs and biological replicates obtained from 2 separate crosses (numbers [_n_] indicated) and compared with the WT average from each group, with significance determined by the Student’s t test. (D) Relative ratio of PC1-NTR to NTS expression for indicated MEF genotypes (numbers [n_] indicated). The significance of the difference between means was compared using the Student’s_t test. For C and D, quartile box plots represent the median, quartiles, and minimum/maximum range, with means of each group in parentheses. **P < 0.01; ***P < 0.001; ****P < 0.0001.
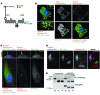
Figure 4. PC1 maturation and trafficking depend on PC2.
(A) IB of membrane-purified proteins from MEFs derived from WT,Pkd2+/–, Pkd2–/–, and_Pkd1–/–_ embryos detected with PC1 NT or PC2 antibodies. A Coomassie-stained loading control is shown. PC1-NTR was completely absent and PC1-NTS elevated in Pkd2–/– cells. Representative blots are shown from 3 independent experiments. (B) Glycosylation analysis of WT and_Pkd2–/–_ MEFs showing that PC1-NTR was absent and PC1-NTS elevated in Pkd2–/– cells compared with WT MEFs. Representative blots are shown from 3 independent experiments. (C) IF detection of cilia (acetylated α-tubulin, Ac. tubulin) and PC2 (H280) in WT,Pkd2–/–, and Pkd1–/– MEFs (scale bar: 10 μm). (D) Quantification of these localizations (n = 50 cilia). PC2 was found on 30% of WT cilia but not on_Pkd2–/–_ or Pkd1–/– cilia, indicating a crucial role for PC1 in PC2 cilia localization in MEFs (****P = 0.0001 by 2-tailed Fisher’s exact test).
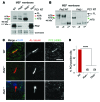
Figure 3. PM and cilia colocalization of PC1 and PC2.
(A) Diagram of mCherry-PC1 and GFP-PC2 fusion proteins used in the IF experiments. (B) Confocal images of mCherry-PC1– and GFP-PC2–cotransfected RCTE cells showing prefixation surface labeling of PC1 (mCherry antibody) in live cells or all PC1 after permeabilization (total mCherry), with PC2 (GFP) and DAPI. Scale bar: 10 μm. Peripheral overlapping PC1/PC2 punctae are indicated with yellow arrows, with colocalization also seen in the ER. (C) Optical sectioning (_z_-stack, XZ plane) of confocal image of ciliated RCTE cells cotransfected with mCherry-PC1 and GFP-PC2 and subjected to prefixation PC1 labeling (mCherry antibodies). Surface PC1 and PC2 colocalized in primary cilia, while PC1 signal was also seen on the PM (red arrow). Scale bar: 10 μm. (D) Low-magnification image of surface-labeled RCTE cells cotransfected with mCherry-PC1 and GFP-PC2, showing mCherry-PC1 detected on the surface only in cells also expressing GFP-PC2 (arrows). Scale bar: 50 μm. (E) Deglycosylation analysis of RCTE cells expressing mCherry-PC1 alone or cotransfected with GFP-PC2. Mature mCherry-PC1 (PC1-NTR) was detected only in cells cotransfected with GFP-PC2, while cleaved ER-resident mCherry-PC1 (NTS) accumulated in the absence of PC2. Representative blots are shown from 3 independent experiments.
Figure 2. Subcellular localization of PC1 glycoforms.
(A) Labeling of RCTE cell surface proteins using alkoxyamine biotin (Alk. Biotin) and IP with neutravidin shows that PC1-NTR was the sole PC1 glycoform localized to the cell surface. PC2 was only detected with neutravidin IP after prolonged exposure and remained sensitive to EndoH digestion; compare level of input and surface protein. EGFR was used as a PM protein control. Representative blots are shown from 3 independent experiments. IF of streptavidin-488–labeled Alk. Biotin–treated and untreated cells shows efficient surface glycoprotein labeling. Scale bar: 20 μm. (B) Density gradient fractionation of RCTE cells shows that a portion of PC1-NTR cofractionated with markers of PM (ORAI/SMO) and cilia (Arl13b/SMO), while most detectable PC2 was distributed in fractions overlapping with ER proteins, calnexin, and STIM1, with minor overlap with the cilia fraction. The relative signal intensity is plotted below. Gradient samples were loaded on 2 different SDS-PAGE gels that were run simultaneously and transferred onto the same membrane for detection. Representative blots are shown from 3 independent experiments. (C) Coimmunoprecipitation of PC2 with PC1 from gradient fraction 12 (cilia enriched) followed by deglycosylation using EndoH (+E) or PNGase F (+P) or no enzyme (Un). Only the EndoH-resistant PC1-NTR glycoform along with EndoH-sensitive PC2 cofractionated with Arl13b.
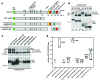
Figure 1. Processing, complexing, and localization of PC1 and PC2.
(A) Immunoblot (IB) of endogenous human PC1 and PC2 derived from membrane fractions of a renal cortical tubule epithelial (RCTE) cell line. Samples were untreated (Un) or treated with EndoH (+E) or PNGase F (+P) and detected with an antibody against N-terminal PC1 (7e12; PC1 NT) or PC2 (YCE2). A nonspecific protein (Supplemental Figure 1, A and B) is indicated (n.s.). N-terminal glycoproducts, EndoH resistant (NTR) and EndoH sensitive (NTS), were resolved and were both reduced to the size of the aa backbone with PNGase F treatment (~330 kDa). All of PC2 was sensitive to EndoH. Representative blots are shown from 3 independent experiments. (B) IPs with a PC1 CT (BD3) or PC2 (YCE2) antibody from RCTE cells followed by deglycosylation detected with PC1 NT or YCE2 (PC2).PKD1–/– epithelial cells (9-12 cells;PKD1–/–) and IP with irrelevant antibody (IgG) were used as negative controls. The PC1 and PC2 complex was formed in the ER (EndoH sensitive), since PC2 coimmunoprecipitated all PC1 glycoforms, including PC1-FL, even in high-salt (500 mM NaCl) conditions. Representative blots are shown from 3 independent experiments. (C) Maturation of PC1-NTR was affected by 2 μg/ml swainsonine (+Sw) treatment. A 72-hour swainsonine treatment reduced the PC1-NTR molecular weight but did not affect PC1-NTS, PC1-FL, or PC2, indicating that only PC1, but not PC2, traffics through the Golgi apparatus. Representative blots are shown from 3 independent experiments. (D) Schematic of PC1 cleavage and glycosylation showing the size of the FL and the 2 GPS/GAIN N-terminal cleavage products, NTS and NTR.
Similar articles
- Identification of polycystin 2 missense mutants targeted for endoplasmic reticulum-associated degradation.
Guerriero CJ, Carattino MD, Sharp KG, Kantz LJ, Gresko NP, Caplan MJ, Brodsky JL. Guerriero CJ, et al. Am J Physiol Cell Physiol. 2025 Feb 1;328(2):C483-C499. doi: 10.1152/ajpcell.00776.2024. Epub 2024 Dec 23. Am J Physiol Cell Physiol. 2025. PMID: 39714991 Free PMC article. - A polycystin-2 (TRPP2) dimerization domain essential for the function of heteromeric polycystin complexes.
Giamarchi A, Feng S, Rodat-Despoix L, Xu Y, Bubenshchikova E, Newby LJ, Hao J, Gaudioso C, Crest M, Lupas AN, Honoré E, Williamson MP, Obara T, Ong AC, Delmas P. Giamarchi A, et al. EMBO J. 2010 Apr 7;29(7):1176-91. doi: 10.1038/emboj.2010.18. Epub 2010 Feb 18. EMBO J. 2010. PMID: 20168298 Free PMC article. - Polycystin-1 negatively regulates Polycystin-2 expression via the aggresome/autophagosome pathway.
Cebotaru V, Cebotaru L, Kim H, Chiaravalli M, Boletta A, Qian F, Guggino WB. Cebotaru V, et al. J Biol Chem. 2014 Mar 7;289(10):6404-6414. doi: 10.1074/jbc.M113.501205. Epub 2014 Jan 23. J Biol Chem. 2014. PMID: 24459142 Free PMC article. - Genetic Mechanisms of ADPKD.
Kim DY, Park JH. Kim DY, et al. Adv Exp Med Biol. 2016;933:13-22. doi: 10.1007/978-981-10-2041-4_2. Adv Exp Med Biol. 2016. PMID: 27730431 Review. - Regulation of polycystin expression, maturation and trafficking.
Hu J, Harris PC. Hu J, et al. Cell Signal. 2020 Aug;72:109630. doi: 10.1016/j.cellsig.2020.109630. Epub 2020 Apr 8. Cell Signal. 2020. PMID: 32275942 Free PMC article. Review.
Cited by
- Synergistic Genetic Interactions between Pkhd1 and Pkd1 Result in an ARPKD-Like Phenotype in Murine Models.
Olson RJ, Hopp K, Wells H, Smith JM, Furtado J, Constans MM, Escobar DL, Geurts AM, Torres VE, Harris PC. Olson RJ, et al. J Am Soc Nephrol. 2019 Nov;30(11):2113-2127. doi: 10.1681/ASN.2019020150. Epub 2019 Aug 19. J Am Soc Nephrol. 2019. PMID: 31427367 Free PMC article. - Co-segregation of candidate polymorphism rs201204878 of the PKD1 gene in a large Iranian family with autosomal dominant polycystic disease.
Ranjzad F, Tara A, Basiri A, Aghdami N, Moghadasali R. Ranjzad F, et al. Exp Ther Med. 2019 Aug;18(2):1345-1349. doi: 10.3892/etm.2019.7693. Epub 2019 Jun 19. Exp Ther Med. 2019. PMID: 31384335 Free PMC article. - GANAB and _N_-Glycans Substrates Are Relevant in Human Physiology, Polycystic Pathology and Multiple Sclerosis: A Review.
De Masi R, Orlando S. De Masi R, et al. Int J Mol Sci. 2022 Jul 1;23(13):7373. doi: 10.3390/ijms23137373. Int J Mol Sci. 2022. PMID: 35806376 Free PMC article. Review. - Recent advances in understanding ion transport mechanisms in polycystic kidney disease.
Sudarikova AV, Vasileva VY, Sultanova RF, Ilatovskaya DV. Sudarikova AV, et al. Clin Sci (Lond). 2021 Nov 12;135(21):2521-2540. doi: 10.1042/CS20210370. Clin Sci (Lond). 2021. PMID: 34751394 Free PMC article. Review. - Human-Specific Abnormal Alternative Splicing of Wild-Type PKD1 Induces Premature Termination of Polycystin-1.
Lea WA, Parnell SC, Wallace DP, Calvet JP, Zelenchuk LV, Alvarez NS, Ward CJ. Lea WA, et al. J Am Soc Nephrol. 2018 Oct;29(10):2482-2492. doi: 10.1681/ASN.2018040442. Epub 2018 Sep 5. J Am Soc Nephrol. 2018. PMID: 30185468 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK090728/DK/NIDDK NIH HHS/United States
- T32 DK007013/DK/NIDDK NIH HHS/United States
- DK090728/DK/NIDDK NIH HHS/United States
- R01 DK080688/DK/NIDDK NIH HHS/United States
- R01-DK058816/DK/NIDDK NIH HHS/United States
- R01 DK058816/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous