Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model - PubMed (original) (raw)
Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model
Julius A Steinbeck et al. Nat Biotechnol. 2015 Feb.
Abstract
Recent studies have shown evidence of behavioral recovery after transplantation of human pluripotent stem cell (PSC)-derived neural cells in animal models of neurological disease. However, little is known about the mechanisms underlying graft function. Here we use optogenetics to modulate in real time electrophysiological and neurochemical properties of mesencephalic dopaminergic (mesDA) neurons derived from human embryonic stem cells (hESCs). In mice that had recovered from lesion-induced Parkinsonian motor deficits, light-induced selective silencing of graft activity rapidly and reversibly re-introduced the motor deficits. The re-introduction of motor deficits was prevented by the dopamine agonist apomorphine. These results suggest that functionality depends on graft neuronal activity and dopamine release. Combining optogenetics, slice electrophysiology and pharmacological approaches, we further show that mesDA-rich grafts modulate host glutamatergic synaptic transmission onto striatal medium spiny neurons in a manner reminiscent of endogenous mesDA neurons. Thus, application of optogenetics in cell therapy can link transplantation, animal behavior and postmortem analysis to enable the identification of mechanisms that drive recovery.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures
Figure 1
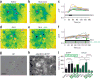
In vitro immunocytochemical characterization of opsin-expressing hESC lines and dopaminergic progeny. Upper panels, hSyn-eNpHR3.0-EYFP (HALO) line, lower panels, hSyn-EYFP (EYFP) line. (a) Transgene harboring clonal hESC lines expressed OCT4 (red). (b) By day (D) 30, >98% of all TUJ+ neurons (red) expressed HALO/EYFP. (c) At D30, >98% of all TH+ (red)/NURR1+ (blue) neurons expressed HALO/EYFP. (d) Confocal imaging shows HALO localization in TH+/NURR1+ neurons largely confined to membranes and processes. (e) Quantification and comparison of transgene expression in all neurons (t = 0.18, P = 0.86, N.S.) and TH+ neurons (t = 0.32, P = 0.76, N.S.) between the HALO and EYFP line. Scale bar, 50 μm. Error bars represent s.e.m.
Figure 2
In vitro physiologic and neurochemical assessment of optogenetic control. (a) Representative ratiometric image of a D90, HALO-expressing, mesDA-rich culture after incubation with Fura-2. (b,c) A glutamate pulse (GLU, 100 μM) generates a calcium response (b), quantified in c. (d–f) During continuous glutamate perfusion (GLU, 50 μM, d) 550-nm light pulses (e) generate inactivating calcium signals in soma and dendrites of neurons, quantified in f. (g,h) Bright-field (g) and eNpHR3.0-EYFP expression (h) of the same region. Scale bar, 20 μm. (i) HPLC measurements of dopamine (DA) release from 90- to 100-day-old cultures after exposure to various stimuli and in the presence or absence of light-induced neuronal silencing. KCl (55 mM) and glutamate (GLU, 100 μM) exposure increase dopamine release in both EYFP- (black bars) and HALO- (green bars) expressing dopamine neurons by about twofold. Optogenetic inhibition at 543 nm (bars with bright green border) reduced dopamine release from HALO-expressing cultures only, in the presence and absence of glutamate stimulation. *P < 0.05, **P < 0.01, ****P < 0.0001.
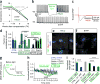
Figure 3
Behavioral, physiological and morphological assessment of graft functional connectivity. (a) Amphetamine-induced rotation assay demonstrates behavioral recovery of grafted animals. (b) Characteristic ~3-Hz pacemaking activity is optogenetically silenced in HALO-expressing neurons only. (c) Evoked postsynaptic potentials (EPSPs) in grafted HALO-expressing neurons following local electrical stimulation are blocked by AMPA-receptor-antagonist NBQX. (d) In the corridor test, unlesioned and nongrafted animals (CTRL, blue bars) did not show lateralized behavior regardless of illumination (green border). Before transplantation, lesioned animals (LES.) showed a strong preference for food retrieval on the side ipsilateral to the lesion, which was inverted by the dopamine agonist apomorphine (LES. + APO, green bar). Animals with EYFP grafts (black bars POST TX) recovered from lesion-induced motor deficiency and were insensitive to illumination or apomorphine. Animals with HALO grafts (green bars POST TX) also recovered from the lesion but reverted to lateralized behavior during optogenetic graft silencing, which was reversed by prior apomorphine injection. Significance was calculated using one-way ANOVA (F (10, 44) = 29.99, P < 0.0001). Adjusted P values indicated in the graph are calculated using Sidak’s multiple comparisons test. (e,f) Confocal analysis of human axons staining for human neurofilament (hNF) and human synaptophysin (hSyn) extend from HALO and EYFP grafts into the host striatum and are in close contact with host DARPP32+ MSNs. Scale bar, 10 μm. (g) Representative examples of electrically evoked AMPA EPSPs recorded from a MSN before and during optogenetic graft silencing in a HALO animal. (h) EPSP amplitudes in MSNs were modulated by optogenetic inhibition of HALO- but not EYFP-expressing grafted neurons or in CTRL animals. (i) EPSPs recorded from MSNs in CTRL, lesioned, EYFP or HALO striata in the presence of optogenetic graft silencing or D1 receptor blockade with 2 μM SCH39166. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Similar articles
- Efficiently Specified Ventral Midbrain Dopamine Neurons from Human Pluripotent Stem Cells Under Xeno-Free Conditions Restore Motor Deficits in Parkinsonian Rodents.
Niclis JC, Gantner CW, Alsanie WF, McDougall SJ, Bye CR, Elefanty AG, Stanley EG, Haynes JM, Pouton CW, Thompson LH, Parish CL. Niclis JC, et al. Stem Cells Transl Med. 2017 Mar;6(3):937-948. doi: 10.5966/sctm.2016-0073. Epub 2016 Oct 14. Stem Cells Transl Med. 2017. PMID: 28297587 Free PMC article. - Illuminating Parkinson's therapy with optogenetics.
Chen Y, Xiong M, Zhang SC. Chen Y, et al. Nat Biotechnol. 2015 Feb;33(2):149-50. doi: 10.1038/nbt.3140. Nat Biotechnol. 2015. PMID: 25658280 Free PMC article. - Induced dopaminergic neurons: A new promise for Parkinson's disease.
Xu Z, Chu X, Jiang H, Schilling H, Chen S, Feng J. Xu Z, et al. Redox Biol. 2017 Apr;11:606-612. doi: 10.1016/j.redox.2017.01.009. Epub 2017 Jan 16. Redox Biol. 2017. PMID: 28110217 Free PMC article. - Clinical translation of stem cell transplantation in Parkinson's disease.
Lindvall O. Lindvall O. J Intern Med. 2016 Jan;279(1):30-40. doi: 10.1111/joim.12415. Epub 2015 Aug 31. J Intern Med. 2016. PMID: 26332959 Review. - Treatment of Parkinson's disease using cell transplantation.
Lindvall O. Lindvall O. Philos Trans R Soc Lond B Biol Sci. 2015 Oct 19;370(1680):20140370. doi: 10.1098/rstb.2014.0370. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26416681 Free PMC article. Review.
Cited by
- Pluripotent Stem Cell Therapies for Parkinson Disease: Present Challenges and Future Opportunities.
Kim TW, Koo SY, Studer L. Kim TW, et al. Front Cell Dev Biol. 2020 Aug 6;8:729. doi: 10.3389/fcell.2020.00729. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32903681 Free PMC article. Review. - Neurotrophin Signaling and Stem Cells-Implications for Neurodegenerative Diseases and Stem Cell Therapy.
Pramanik S, Sulistio YA, Heese K. Pramanik S, et al. Mol Neurobiol. 2017 Nov;54(9):7401-7459. doi: 10.1007/s12035-016-0214-7. Epub 2016 Nov 5. Mol Neurobiol. 2017. PMID: 27815842 Review. - Task Force Paper On Cerebellar Transplantation: Are We Ready to Treat Cerebellar Disorders with Cell Therapy?
Cendelin J, Buffo A, Hirai H, Magrassi L, Mitoma H, Sherrard R, Vozeh F, Manto M. Cendelin J, et al. Cerebellum. 2019 Jun;18(3):575-592. doi: 10.1007/s12311-018-0999-1. Cerebellum. 2019. PMID: 30607797 Review. - Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior.
Palma-Tortosa S, Tornero D, Grønning Hansen M, Monni E, Hajy M, Kartsivadze S, Aktay S, Tsupykov O, Parmar M, Deisseroth K, Skibo G, Lindvall O, Kokaia Z. Palma-Tortosa S, et al. Proc Natl Acad Sci U S A. 2020 Apr 21;117(16):9094-9100. doi: 10.1073/pnas.2000690117. Epub 2020 Apr 6. Proc Natl Acad Sci U S A. 2020. PMID: 32253308 Free PMC article. - Plasticity of Synaptic Transmission in Human Stem Cell-Derived Neural Networks.
Dong Y, Xiong M, Chen Y, Tao Y, Li X, Bhattacharyya A, Zhang SC. Dong Y, et al. iScience. 2020 Feb 21;23(2):100829. doi: 10.1016/j.isci.2020.100829. Epub 2020 Jan 9. iScience. 2020. PMID: 31981924 Free PMC article.
References
- Kirkeby A, et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Reports. 2012;1:703–714. - PubMed
- Roy NS, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. - PubMed
- Tornero D, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136:3561–3577. - PubMed
- Dunnett SB, Hernandez TD, Summerfield A, Jones GH, Arbuthnott G. Graft-derived recovery from 6-OHDA lesions: specificity of ventral mesencephalic graft tissues. Exp Brain Res. 1988;71:411–424. - PubMed
Publication types
MeSH terms
Grants and funding
- P01 DA010154/DA/NIDA NIH HHS/United States
- R01 NS052671/NS/NINDS NIH HHS/United States
- NS052671/NS/NINDS NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 NS075222/NS/NINDS NIH HHS/United States
- R01 AA019801/AA/NIAAA NIH HHS/United States
- NS075222/NS/NINDS NIH HHS/United States
- T32 MH015174/MH/NIMH NIH HHS/United States
- R01 DA007418/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical