Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria - PubMed (original) (raw)
Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria
Carla Pérez-Cruz et al. PLoS One. 2015.
Abstract
Outer-inner membrane vesicles (O-IMVs) were recently described as a new type of membrane vesicle secreted by the Antarctic bacterium Shewanella vesiculosa M7T. Their formation is characterized by the protrusion of both outer and plasma membranes, which pulls cytoplasmic components into the vesicles. To demonstrate that this is not a singular phenomenon in a bacterium occurring in an extreme environment, the identification of O-IMVs in pathogenic bacteria was undertaken. With this aim, a structural study by Transmission Electron Microscopy (TEM) and Cryo-transmission electron microscopy (Cryo-TEM) was carried out, confirming that O-IMVs are also secreted by Gram-negative pathogenic bacteria such as Neisseria gonorrhoeae, Pseudomonas aeruginosa PAO1 and Acinetobacter baumannii AB41, in which they represent between 0.23% and 1.2% of total vesicles produced. DNA and ATP, which are components solely found in the cell cytoplasm, were identified within membrane vesicles of these strains. The presence of DNA inside the O-IMVs produced by N. gonorrhoeae was confirmed by gold DNA immunolabeling with a specific monoclonal IgM against double-stranded DNA. A proteomic analysis of N. gonorrhoeae-derived membrane vesicles identified proteins from the cytoplasm and plasma membrane. This confirmation of O-IMV extends the hitherto uniform definition of membrane vesicles in Gram-negative bacteria and explains the presence of components in membrane vesicles such as DNA, cytoplasmic and inner membrane proteins, as well as ATP, detected for the first time. The production of these O-IMVs by pathogenic Gram-negative bacteria opens up new areas of study related to their involvement in lateral gene transfer, the transfer of cytoplasmic proteins, as well as the functionality and role of ATP detected in these new vesicles.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. MVs visualized by TEM in pathogenic bacteria.
HPF-FS sections correspond to the three pathogenic strains grown in solid media: (A) N. gonorrhoeae, (B) Pseudomonas PAO1 and (C) A. baumannii. O-IMVs (marked with arrows) are observed in the extracellular matter of the three pathogenic bacteria. O-IMVs in A and B clearly show a double bilayer, which exhibits the same staining profile as OM and PM from the respective cells. The O-IMV inner membrane encloses a material similar to that seen in the cytoplasm of the respective cells. OM: outer membrane; PM: plasma membrane; CC: cytoplasmic content. Bars 200 nm.
Figure 2. An O-IMV being released from the surface of a N. gonorrhoeae cell.
(A) The TEM micrograph provides a view of an O-IMV at the moment of its formation, where the outer membrane (OM) is being extruded, dragging along the plasma membrane (PM) and a portion of the cytoplasmic content (CC). (B) The same image as A but with the cell envelope outlined to highlight the formation and structure of the O-IMV. Bars, 200 nm.
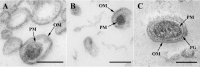
Figure 3. O-IMV visualized by TEM.
TEM micrographs from HPF-FS sections of MVs isolated from (A) N. gonorrhoeae, (B) Pseudomonas PAO1 and (C) A. baumannii. O-IMVs observed in MV preparations from the three strains have certain features in common: all are surrounded by an external bilayer, probably corresponding to the outer membrane (OM) of the cell, and contain an inner membrane, probably corresponding to the plasma membrane (PM) of the cell, which entraps a high electron-dense material. In the image of O-IMVs from A. baumannii the putative peptidoglican layer (PG) can be seen. Bars 100 nm.
Figure 4. Cryo-TEM visualization of O-IMVs in pathogenic bacteria.
Cryo-electron micrographs showing whole plunge-frozen cells from three pathogenic bacteria, and their derived O-IMVs: (A) N. gonorrhoeae, (B) Pseudomonas PAO1, and (C) A. baumannii. Whole cells with well-defined envelopes are observed in A and B (large black squares show a magnified area of cell envelopes). The new O-IMVs in the three analyzed samples exhibit the same double layer as cells, and are filled with an electron-dense material similar to that seen in the cell cytoplasm (large white squares show a magnified area of the O-IMV). Conventional OMVs are also visualized in images A and C (black arrows). OM: Outer Membrane; PM: Plasma membrane; PG: Peptidoglycan. Bars, 500 nm (A, C) and 250 nm (B).
Figure 5. Quantification of O-IMVs on thin frozen foils from the total MVs using Cryo-TEM.
(A) Overview of a thin frozen foil obtained from A. baumannii. _S_ingle-layer vesicles are highly abundant, while double-layer O-IMVs can be observed in all the tracked fields, but much less often (white arrows). (B) Cryo-TEM images of thin frozen foils from the MVs of the three assayed strains. Both types of vesicles are observed, the single-layer OMVs and the new double-layer O-IMVs (white arrows). In A. baumannii O-IMVs, the presence of the putative intact peptidoglycan layer is also observed (PG). Bars 500 nm (A) and 200 nm (B).
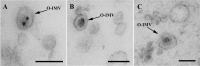
Figure 6. DNA gold immunolabeling on Lowicryl HM20 thin sections of HPF-FS isolated MVs from N. gonorrhoeae.
(A) TEM micrograph showing an O-IMV immunolabeled with a monoclonal IgM specific against dsDNA and a secondary goat anti-mouse antibody coupled to 12-nm colloidal gold. The gold mark is localized inside the inner layer that contains the electron-dense material, which confirms that the DNA is packaged within the new O-IMV. (B) TEM micrograph of MVs labeled only with the secondary antibody. (C) TEM micrograph of MVs from grids preincubated with 1 mg/ml DNase I and then immunolabeled with the anti-dsDNA IgM and a secondary antibody coupled to gold. Bars, 100 nm.
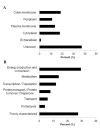
Figure 7. Protein content from the N. _gonorrhoeae_-derived MVs.
(A) Distribution of proteins identified from the total MVs from N. gonorrhoeae based on their subcellular location. (B) Functional classification of the 51 proteins predicted to be localized in the cytoplasm and plasma membrane of N. gonorrhoeae cells.
Similar articles
- Phage-Mediated Explosive Cell Lysis Induces the Formation of a Different Type of O-IMV in Shewanella vesiculosa M7T.
Baeza N, Delgado L, Comas J, Mercade E. Baeza N, et al. Front Microbiol. 2021 Oct 8;12:713669. doi: 10.3389/fmicb.2021.713669. eCollection 2021. Front Microbiol. 2021. PMID: 34690958 Free PMC article. - New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content.
Pérez-Cruz C, Carrión O, Delgado L, Martinez G, López-Iglesias C, Mercade E. Pérez-Cruz C, et al. Appl Environ Microbiol. 2013 Mar;79(6):1874-81. doi: 10.1128/AEM.03657-12. Epub 2013 Jan 11. Appl Environ Microbiol. 2013. PMID: 23315742 Free PMC article. - Increased production of outer membrane vesicles by cultured freshwater bacteria in response to ultraviolet radiation.
Gamalier JP, Silva TP, Zarantonello V, Dias FF, Melo RC. Gamalier JP, et al. Microbiol Res. 2017 Jan;194:38-46. doi: 10.1016/j.micres.2016.08.002. Epub 2016 Nov 3. Microbiol Res. 2017. PMID: 27938861 - Biogenesis of the Gram-negative bacterial outer membrane.
Bos MP, Tommassen J. Bos MP, et al. Curr Opin Microbiol. 2004 Dec;7(6):610-6. doi: 10.1016/j.mib.2004.10.011. Curr Opin Microbiol. 2004. PMID: 15556033 Review.
Cited by
- Biopearling of Interconnected Outer Membrane Vesicle Chains by a Marine Flavobacterium.
Fischer T, Schorb M, Reintjes G, Kolovou A, Santarella-Mellwig R, Markert S, Rhiel E, Littmann S, Becher D, Schweder T, Harder J. Fischer T, et al. Appl Environ Microbiol. 2019 Sep 17;85(19):e00829-19. doi: 10.1128/AEM.00829-19. Print 2019 Oct 1. Appl Environ Microbiol. 2019. PMID: 31324630 Free PMC article. - Bacterial Outer Membrane Vesicles and Immune Modulation of the Host.
Charpentier LA, Dolben EF, Hendricks MR, Hogan DA, Bomberger JM, Stanton BA. Charpentier LA, et al. Membranes (Basel). 2023 Aug 24;13(9):752. doi: 10.3390/membranes13090752. Membranes (Basel). 2023. PMID: 37755174 Free PMC article. Review. - Phage-Mediated Explosive Cell Lysis Induces the Formation of a Different Type of O-IMV in Shewanella vesiculosa M7T.
Baeza N, Delgado L, Comas J, Mercade E. Baeza N, et al. Front Microbiol. 2021 Oct 8;12:713669. doi: 10.3389/fmicb.2021.713669. eCollection 2021. Front Microbiol. 2021. PMID: 34690958 Free PMC article. - Gene Transfer Potential of Outer Membrane Vesicles of Gram-Negative Bacteria.
Dell'Annunziata F, Folliero V, Giugliano R, De Filippis A, Santarcangelo C, Izzo V, Daglia M, Galdiero M, Arciola CR, Franci G. Dell'Annunziata F, et al. Int J Mol Sci. 2021 Jun 1;22(11):5985. doi: 10.3390/ijms22115985. Int J Mol Sci. 2021. PMID: 34205995 Free PMC article. Review. - Characterization of membrane vesicles in Alteromonas macleodii indicates potential roles in their copiotrophic lifestyle.
Fadeev E, Carpaneto Bastos C, Hennenfeind JH, Biller SJ, Sher D, Wietz M, Herndl GJ. Fadeev E, et al. Microlife. 2022 Dec 20;4:uqac025. doi: 10.1093/femsml/uqac025. eCollection 2023. Microlife. 2022. PMID: 37223730 Free PMC article.
References
- Kuehn MJ, Kesty NC (2005) Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev 19: 2645–2655. - PubMed
- Mashburn-Warren LM, Whiteley M (2006) Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol 61: 839–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work received funding from the following sources: Government of Spain (CICYT grant CTQ 2010-21183-C02-01/PPQ)EM CP-C, Autonomous Government of Catalonia (grant 2009SGR1212)EM CL-I, and Fellowship FFAR2012.3 from the University of Barcelona (CP-C). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources