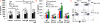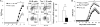Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation - PubMed (original) (raw)
Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation
Sheng Xiao et al. J Immunol. 2015.
Abstract
T cell Ig and mucin domain (Tim)-1 identifies IL-10-producing regulatory B cells (Bregs). Mice on the C57BL/6 background harboring a loss-of-function Tim-1 mutant showed progressive loss of IL-10 production in B cells and with age developed severe multiorgan tissue inflammation. We demonstrate that Tim-1 expression and signaling in Bregs are required for optimal production of IL-10. B cells with Tim-1 defects have impaired IL-10 production but increased proinflammatory cytokine production, including IL-1 and IL-6. Tim-1-deficient B cells promote Th1 and Th17 responses but inhibit the generation of regulatory T cells (Foxp3(+) and IL-10-producing type 1 regulatory T cells) and enhance the severity of experimental autoimmune encephalomyelitis. Mechanistically, Tim-1 on Bregs is required for apoptotic cell (AC) binding to Bregs and for AC-induced IL-10 production in Bregs. Treatment with ACs reduces the severity of experimental autoimmune encephalomyelitis in hosts with wild-type but not Tim-1-deficient Bregs. Collectively, these findings suggest that in addition to serving as a marker for identifying IL-10-producing Bregs, Tim-1 is also critical for maintaining self-tolerance by regulating IL-10 production in Bregs.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures
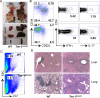
Figure 1. Tim-1Δmucin mice at 16-18+ months of age develop splenomegaly and multi-organ inflammation
A) Representative images of organs from 16-18+-month old WT and Tim-1Δmucin mice. B&C) Representative flow cytometry plots showing CD4+ T cell phenotypes in spleens (B) and livers (C) isolated from 16-18+-month old WT and Tim-1Δmucin mice (n = 10). D) Representative histopathology of livers and lungs from 16-18+-month-old WT and Tim-1Δmucin mice. There are massive mononuclear cell infiltrates in the Tim-1Δmucin mice. Hematoxylin and eosin stain, 15x.
Figure 2. Tim-1 and BCR or IL-21 signaling together strongly promoted B cell IL-10 production while a defect in Tim-1 signaling in B cells reduced IL-10 production
Purified splenic CD19+ B cells from 2-3 month-old WT, Tim-1Δmucin or Tim-1−/− mice were cultured in the presence of anti-Tim-1 (clone 5F12), (Fab’)2 fragment anti-IgM or both without (A) or with IL-21 (B). After 3 days, IL-10 production in culture supernatants was measured by ELISA. * P < 0.01; ns, not significant. C) Representative flow cytometry plots showing Tim-1 expression by splenic CD19+ B cells from WT and Tim-1−/− mice after 3-day culture in the presence of IL-21. n ≥ 3 per group.
Figure 3. Tim-1 expression or defects affects the balance between regulatory and inflammatory cytokines in B cells that subsequently alter T cell responses
A) Purified splenic CD19+ B cells from WT or Tim-1−/− mice were cultured in the presence of anti-IgM ((Fab’)2 fragment) for 24 h. Total RNA was isolated, and relative expression (mean ± SEM; n = 5) of Tim-1, IL10, IL12, IL6, and IL1b mRNA was measured by realtime PCR. * P < 0.01. B) WT total CD4+ T cells (10 x 106/mouse) were co-transferred together with WT or Tim-1Δmucin CD19+ B cells (20 x 106) into Rag1−/− mice. One day after, mice were immunized with MOG35-55/CFA to induce EAE. At the peak of disease, splenic Tim-1+ and Tim-1− CD19+ B cells were purified from WT and Tim-1Δmucin groups of mice. Total RNA was isolated, and relative expression (mean + SEM; n = 5) of Tim-1, IL10, IL12, IL6, and IL1b mRNA was measured by realtime PCR. * P < 0.01. C) WT naïve CD4+ T cells were cultured with splenic CD19+ B cells purified from WT or Tim-1−/− IL-10GFP/+ mice in the presence of anti-CD3 under Th0 (no cytokine), Th1 (IL-12 + anti-IL-4), Th2 (IL-4 + anti-IL-12/anti-IFN-γ), Th17 (TGF-β1 + IL-6), Tr1 (TGF-β1 + IL-27), and iTreg (TGF-β1) conditions. After culture for 4 days, production of indicated cytokines in T cells and IL-10 (GFP+) in B cells was measured by flow cytometry after intracellular cytokine staining. Representative of 5 independent experiments was shown. D) WT CD4+ naïve T cells were cultured with Tim-1+ or Tim-1− B cells purified from WT in the presence of anti-CD3 under Th17, Tr1, and iTreg conditions. After culture for 4 days, production of indicated cytokines in T cells was measured by flow cytometry after intracellular cytokine staining. Representative data from 3 independent experiments are shown.
Figure 4. Effect of Tim-1 expression or defects in B cells on EAE and T cell responses
A) WT total CD4+ T cells (10 × 106/mouse) were co-transferred together with either WT or Tim-1−/− CD19+ B cells (20 × 106) into Rag1−/− mice; the recipients were then immunized with MOG35-55/CFA to induce EAE. Mice were scored daily for clinical signs of EAE (left panel; n =10 per group). * P < 0.05. On day 20, CNS-infiltrating mononuclear cells were isolated and examined for the frequencies of IFN-γ+, IL-17+, and Foxp3+ cells in CD4+ T cell gates by flow cytometry after intracellular staining (middle panel; n = 5). CNS-infiltrating CD4+ T cells were also isolated and measured for their IL10 mRNA expression by realtime PCR (right panel; ** P < 0.01; n = 4 per group). B) WT total CD4+ T cells (10 × 106) were co-transferred together with WT (20 × 106), Tim-1-−/− (20 × 106), or Tim-1−/− (20 × 106) plus WT Tim-1+ (2 × 106) B cells into Rag1−/− mice; the recipients were then immunized with MOG35-55/CFA to induce EAE. Mice (n = 8-10 per group) were scored daily for clinical signs of EAE. * P < 0.05.
Figure 5. Effect of apoptotic cells on WT and Tim-1−/− B cells and the development of EAE
A) WT, Tim-1Δmucin and Tim-1−/− B cells from 2-4 month-old mice were incubated with CMFDA-labeled apoptotic WT thymocytes (AC) for 30 min, and analyzed by flow cytometry. WT and Tim-1Δmucin B cells had comparable Tim-1 expression. Gating strategy for Tim-1 staining is shown in the left panel. n = 3-5 per group. B) WT and Tim-1−/− B cells were cultured with unlabeled AC for 3 days, and IL-10 production in culture supernatants was then measured by ELISA. * P < 0.001; ns, not significant; n = 5. C) WT total CD4+ T cells (10 × 106/mouse) were co-transferred together with either WT or Tim-1−/− CD19+ B cells (20 × 106) into Rag1−/− mice. Apoptotic WT thymocytes (30 × 106/mouse) were injected one day before immunization with MOG35-55/CFA for EAE induction. Mice (n = 8 per group) were scored daily for clinical signs of EAE. * P < 0.05.
Similar articles
- Characterization and Activity of TIM-1 and IL-10-Reporter Expressing Regulatory B Cells.
Mohib K, Rothstein DM, Ding Q. Mohib K, et al. Methods Mol Biol. 2021;2270:179-202. doi: 10.1007/978-1-0716-1237-8_10. Methods Mol Biol. 2021. PMID: 33479899 - Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice.
Xiao S, Brooks CR, Zhu C, Wu C, Sweere JM, Petecka S, Yeste A, Quintana FJ, Ichimura T, Sobel RA, Bonventre JV, Kuchroo VK. Xiao S, et al. Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):12105-10. doi: 10.1073/pnas.1120914109. Epub 2012 Jul 5. Proc Natl Acad Sci U S A. 2012. PMID: 22773818 Free PMC article. - TIM-1 defines a human regulatory B cell population that is altered in frequency and function in systemic sclerosis patients.
Aravena O, Ferrier A, Menon M, Mauri C, Aguillón JC, Soto L, Catalán D. Aravena O, et al. Arthritis Res Ther. 2017 Jan 19;19(1):8. doi: 10.1186/s13075-016-1213-9. Arthritis Res Ther. 2017. PMID: 28103916 Free PMC article. - Regulatory B cells: TIM-1, transplant tolerance, and rejection.
Cherukuri A, Mohib K, Rothstein DM. Cherukuri A, et al. Immunol Rev. 2021 Jan;299(1):31-44. doi: 10.1111/imr.12933. Epub 2021 Jan 22. Immunol Rev. 2021. PMID: 33484008 Free PMC article. Review. - The role of B regulatory (B10) cells in inflammatory disorders and their potential as therapeutic targets.
Wu H, Su Z, Barnie PA. Wu H, et al. Int Immunopharmacol. 2020 Jan;78:106111. doi: 10.1016/j.intimp.2019.106111. Epub 2019 Dec 24. Int Immunopharmacol. 2020. PMID: 31881524 Review.
Cited by
- The current understanding of the phenotypic and functional properties of human regulatory B cells (Bregs).
Ahsan NF, Lourenço S, Psyllou D, Long A, Shankar S, Bashford-Rogers R. Ahsan NF, et al. Oxf Open Immunol. 2024 Sep 20;5(1):iqae012. doi: 10.1093/oxfimm/iqae012. eCollection 2024. Oxf Open Immunol. 2024. PMID: 39346706 Free PMC article. Review. - Inhibition of the TIM-1 and -3 signaling pathway ameliorates disease in a murine model of rheumatoid arthritis.
Nozaki Y, Akiba H, Akazawa H, Yamazawa H, Ishimura K, Kinoshita K, Matsumura I. Nozaki Y, et al. Clin Exp Immunol. 2024 Sep 16;218(1):55-64. doi: 10.1093/cei/uxae056. Clin Exp Immunol. 2024. PMID: 38975703 - Elevated Serum KIM-1 in Sepsis Correlates with Kidney Dysfunction and the Severity of Multi-Organ Critical Illness.
Brozat JF, Harbalioğlu N, Hohlstein P, Abu Jhaisha S, Pollmanns MR, Adams JK, Wirtz TH, Hamesch K, Yagmur E, Weiskirchen R, Tacke F, Trautwein C, Koch A. Brozat JF, et al. Int J Mol Sci. 2024 May 27;25(11):5819. doi: 10.3390/ijms25115819. Int J Mol Sci. 2024. PMID: 38892009 Free PMC article. - Human regulatory memory B cells defined by expression of TIM-1 and TIGIT are dysfunctional in multiple sclerosis.
Varghese JF, Kaskow BJ, von Glehn F, Case J, Li Z, Julé AM, Berdan E, Ho Sui SJ, Hu Y, Krishnan R, Chitnis T, Kuchroo VK, Weiner HL, Baecher-Allan CM. Varghese JF, et al. Front Immunol. 2024 Apr 30;15:1360219. doi: 10.3389/fimmu.2024.1360219. eCollection 2024. Front Immunol. 2024. PMID: 38745667 Free PMC article. - Regulatory B Cells in Solid Organ Transplantation: From Immune Monitoring to Immunotherapy.
Elias C, Chen C, Cherukuri A. Elias C, et al. Transplantation. 2024 May 1;108(5):1080-1089. doi: 10.1097/TP.0000000000004798. Epub 2023 Oct 2. Transplantation. 2024. PMID: 37779239 Review.
References
- DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. - PubMed
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. - PubMed
- Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends Immunol. 2013;34:169–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS030843/NS/NINDS NIH HHS/United States
- P01NS076410/NS/NINDS NIH HHS/United States
- K01 DK099473/DK/NIDDK NIH HHS/United States
- K01 DK090105/DK/NIDDK NIH HHS/United States
- K01DK090105/DK/NIDDK NIH HHS/United States
- P01 NS076410/NS/NINDS NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R01NS030843/NS/NINDS NIH HHS/United States
- P01AI039671/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases