An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence - PubMed (original) (raw)
An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence
TuAnh Ngoc Huynh et al. Proc Natl Acad Sci U S A. 2015.
Abstract
The nucleotide cyclic di-3',5'- adenosine monophosphate (c-di-AMP) was recently identified as an essential and widespread second messenger in bacterial signaling. Among c-di-AMP-producing bacteria, altered nucleotide levels result in several physiological defects and attenuated virulence. Thus, a detailed molecular understanding of c-di-AMP metabolism is of both fundamental and practical interest. Currently, c-di-AMP degradation is recognized solely among DHH-DHHA1 domain-containing phosphodiesterases. Using chemical proteomics, we identified the Listeria monocytogenes protein PgpH as a molecular target of c-di-AMP. Biochemical and structural studies revealed that the PgpH His-Asp (HD) domain bound c-di-AMP with high affinity and specifically hydrolyzed this nucleotide to 5'-pApA. PgpH hydrolysis activity was inhibited by ppGpp, indicating a cross-talk between c-di-AMP signaling and the stringent response. Genetic analyses supported coordinated regulation of c-di-AMP levels in and out of the host. Intriguingly, a L. monocytogenes mutant that lacks c-di-AMP phosphodiesterases exhibited elevated c-di-AMP levels, hyperinduced a host type-I IFN response, and was significantly attenuated for infection. Furthermore, PgpH homologs, which belong to the 7TMR-HD family, are widespread among hundreds of c-di-AMP synthesizing microorganisms. Thus, PgpH represents a broadly conserved class of c-di-AMP phosphodiesterase with possibly other physiological functions in this crucial signaling network.
Keywords: HD domain; Listeria monocytogenes; bacterial signal transduction; c-di-AMP; phosphodiesterase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
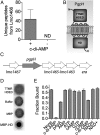
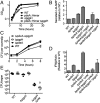
Identification and characterization of PgpH and c-di-AMP interactions. (A) Spectral counting of Lmo1466 signature peptides interacting with c-di-AMP Sepharose beads (+) and control (-) beads. ND, none detected. Error bars represent the SEM of spectral counts from three independent experiments. (B) Schematic diagram of Lmo1466 domain organization: extracellular domain (7TMR-HDED), seven transmembrane helices, and cytoplasmic HD domain (HD). (C) Operon organization surrounding pgpH. (D) Nucleotide binding assays (DRACALA) for 32P-c-di-AMP with PgpH extracellular domain (7TMR HDED; 220 μM), MBP-HD domain (7 μM), and maltose binding protein (MBP, 21 μM) as a control. (E) Binding of PgpH HD domain with 32P-c-di-AMP in the presence of competing unlabeled nucleotides, each at 200 μM concentration. For binding assays, error bars represent the SEM of duplicate measures and are representative of two independent experiments.
Fig. 2.
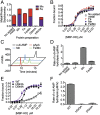
Metal cations and the HD motif are required for c-di-AMP binding and hydrolysis by PgpH HD domain. PgpH HD domain was purified as an MBP-HD construct with different metal salts (0.35 mM in total when added to expression cultures) and analyzed for stoichiometry of bound metal (error bars represent average of two measurements) (A); binding affinity with 32P-c-di-AMP (error bars represent two independent experiments, each performed in duplicates) (B); and c-di-AMP hydrolysis activity by HPLC (C) and LC-MS/MS (D). The H543A and D544A mutants were purified with added MnCl2 and analyzed for stoichiometry of bound metal (A), binding affinity with 32P -c-di-AMP (E), and c-di-AMP hydrolysis (F) by LC-MS/MS. LC-MS/MS data are representative of two independent experiments, and error bars represent the SE derived from fitting reaction rates by linear regression.
Fig. 3.
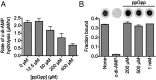
PgpH hydrolysis of c-di-AMP is inhibited by ppGpp. (A) The rate of c-di-AMP hydrolysis in the presence of ppGpp monitored by LC-MS/MS. Error bars are the SE derived from fitting reaction rates by linear regression. (B) Binding of PgpH HD domain with 32P-c-di-AMP in the presence of competing unlabeled c-di-AMP (200 μM) or ppGpp (200 μM–1 mM). Error bars represent the SEM of two measurements.
Fig. 4.
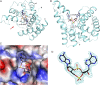
Crystal structure of the HD domain of PgpH in complex with c-di-AMP. (A) Schematic drawing of the structure of PgpH HD domain (in cyan) in complex with c-di-AMP (stick model with carbon atoms in black). The two metal ions in the active site are shown as pink spheres and labeled. The two nucleotides of c-di-AMP are labeled 1 and 2. (B) Structure of the PgpH HD domain in complex with c-di-AMP, viewed along the red arrow in A. (C) Molecular surface of the PgpH HD domain near the c-di-AMP binding site, colored by the electrostatic potential (red, negative; blue, positive). (D) Omit _F_o–_F_c electron density map for c-di-AMP at 2.1 Å resolution, contoured at 2.5σ.
Fig. 5.
Binding mode of c-di-AMP in PgpH HD domain. (A) Detailed interactions between c-di-AMP and the PgpH HD domain. Hydrogen-bonding interactions are indicated with dashed lines (in red) and liganding interactions as thin lines (in gray). The bridging water between the two metal ions is shown as a red sphere and labeled W. (B) Sequence conservation of the PgpH HD domain among the top 250 closest homologs identified by BLAST. Sequence logo was generated by using Jalview. (C) Overlay of the structure of the PgpH HD domain in complex with c-di-AMP (in color) with that of the HD-GYP domain in complex with c-di-GMP (in gray) (29). A large difference in the position of the scissile phosphate group in the c-di-GMP structure is indicated with the blue arrow. All structure figures were produced with PyMOL (
).
Fig. 6.
Characterization of L. monocytogenes c-di-AMP phosphodiesterase mutants. (A) Growth curves of L. monocytogenes strain in chemically defined minimal medium. (B) c-di-AMP secretion by various L. monocytogenes strains in chemically defined minimal medium. Secreted c-di-AMP is normalized to WT. (C) Growth curves in bone marrow-derived macrophages. (D) IFN-β expression by qPCR at 4 h after infection, normalized to WT-infected macrophages. (E) Female C57BL/6 mice (6–8 wk old) were infected with 1 × 105 cfu of the indicated strains. Organs were harvested at 48 hpi, and bacterial burden per spleen (filled circles) and liver (open circles) was enumerated. Median values are presented as horizontal lines. Error bars represent the SEM and are representative of at least two independent experiments.
Fig. 7.
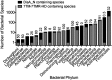
Taxonomic distribution of di-adenylate cyclase and PgpH proteins. The number of DisA_N and 7TM_7TMR_HD containing organisms are reported based on bacterial phylum. Numbers above each bar represent the percentage of DisA_N-containing organisms within a phylum that also harbor a PgpH homolog.
Comment in
- Chemical proteomics reveals a second family of cyclic-di-AMP hydrolases.
Helmann JD. Helmann JD. Proc Natl Acad Sci U S A. 2015 Feb 17;112(7):1921-2. doi: 10.1073/pnas.1500077112. Epub 2015 Jan 30. Proc Natl Acad Sci U S A. 2015. PMID: 25637595 Free PMC article. No abstract available.
Similar articles
- NrnA Is a Linear Dinucleotide Phosphodiesterase with Limited Function in Cyclic Dinucleotide Metabolism in Listeria monocytogenes.
Gall AR, Hsueh BY, Siletti C, Waters CM, Huynh TN. Gall AR, et al. J Bacteriol. 2022 Jan 18;204(1):e0020621. doi: 10.1128/JB.00206-21. Epub 2021 Oct 18. J Bacteriol. 2022. PMID: 34662239 Free PMC article. - Too much of a good thing: regulated depletion of c-di-AMP in the bacterial cytoplasm.
Huynh TN, Woodward JJ. Huynh TN, et al. Curr Opin Microbiol. 2016 Apr;30:22-29. doi: 10.1016/j.mib.2015.12.007. Epub 2016 Jan 7. Curr Opin Microbiol. 2016. PMID: 26773214 Free PMC article. Review. - c-di-AMP Accumulation Impairs Muropeptide Synthesis in Listeria monocytogenes.
Massa SM, Sharma AD, Siletti C, Tu Z, Godfrey JJ, Gutheil WG, Huynh TN. Massa SM, et al. J Bacteriol. 2020 Nov 19;202(24):e00307-20. doi: 10.1128/JB.00307-20. Print 2020 Nov 19. J Bacteriol. 2020. PMID: 33020220 Free PMC article. - Making and Breaking of an Essential Poison: the Cyclases and Phosphodiesterases That Produce and Degrade the Essential Second Messenger Cyclic di-AMP in Bacteria.
Commichau FM, Heidemann JL, Ficner R, Stülke J. Commichau FM, et al. J Bacteriol. 2018 Dec 7;201(1):e00462-18. doi: 10.1128/JB.00462-18. Print 2019 Jan 1. J Bacteriol. 2018. PMID: 30224435 Free PMC article. Review.
Cited by
- Intracellular Concentrations of Borrelia burgdorferi Cyclic Di-AMP Are Not Changed by Altered Expression of the CdaA Synthase.
Savage CR, Arnold WK, Gjevre-Nail A, Koestler BJ, Bruger EL, Barker JR, Waters CM, Stevenson B. Savage CR, et al. PLoS One. 2015 Apr 23;10(4):e0125440. doi: 10.1371/journal.pone.0125440. eCollection 2015. PLoS One. 2015. PMID: 25906393 Free PMC article. - Cyclic Dinucleotide-Controlled Regulatory Pathways in Streptomyces Species.
Tschowri N. Tschowri N. J Bacteriol. 2016 Jan 1;198(1):47-54. doi: 10.1128/JB.00423-15. J Bacteriol. 2016. PMID: 26216850 Free PMC article. Review. - Cyclic di-AMP targets the cystathionine beta-synthase domain of the osmolyte transporter OpuC.
Huynh TN, Choi PH, Sureka K, Ledvina HE, Campillo J, Tong L, Woodward JJ. Huynh TN, et al. Mol Microbiol. 2016 Oct;102(2):233-243. doi: 10.1111/mmi.13456. Epub 2016 Jul 26. Mol Microbiol. 2016. PMID: 27378384 Free PMC article. - A STING-based biosensor affords broad cyclic dinucleotide detection within single living eukaryotic cells.
Pollock AJ, Zaver SA, Woodward JJ. Pollock AJ, et al. Nat Commun. 2020 Jul 15;11(1):3533. doi: 10.1038/s41467-020-17228-y. Nat Commun. 2020. PMID: 32669552 Free PMC article. - Comparative analysis of five type II TA systems identified in Pseudomonas aeruginosa reveals their contributions to persistence and intracellular survival.
Song Y, Tang H, Bao R. Song Y, et al. Front Cell Infect Microbiol. 2023 Feb 13;13:1127786. doi: 10.3389/fcimb.2023.1127786. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36844395 Free PMC article.
References
- Kalia D, et al. Nucleotide, c-di-GMP, c-di-AMP, cGMP, cAMP, (p)ppGpp signaling in bacteria and implications in pathogenesis. Chem Soc Rev. 2013;42(1):305–341. - PubMed
- Römling U. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci Signal. 2008;1(33):pe39. - PubMed
- Corrigan RM, Gründling A. Cyclic di-AMP: Another second messenger enters the fray. Nat Rev Microbiol. 2013;11(8):513–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI108698/AI/NIAID NIH HHS/United States
- R01 DK067238/DK/NIDDK NIH HHS/United States
- S10OD012018/OD/NIH HHS/United States
- R01DK067238/DK/NIDDK NIH HHS/United States
- P41 GM111244/GM/NIGMS NIH HHS/United States
- R56AI108698/AI/NIAID NIH HHS/United States
- S10 OD012018/OD/NIH HHS/United States
- R01 AI116669/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases