Chronic fluoxetine increases extra-hippocampal neurogenesis in adult mice - PubMed (original) (raw)
Chronic fluoxetine increases extra-hippocampal neurogenesis in adult mice
Benjamin D Sachs et al. Int J Neuropsychopharmacol. 2014.
Abstract
Background: Chronic treatment with antidepressants has been shown to enhance neurogenesis in the adult mammalian brain. Although this effect was initially reported to be restricted to the hippocampus, recent work has suggested that fluoxetine, a selective serotonin reuptake inhibitor, also promotes neurogenesis in the cortex. However, whether antidepressants target neural progenitor cells in other brain regions has not been examined.
Methods: Here, we used BrdU labeling and immunohistochemistry with a transgenic mouse line in which nestin+ neural progenitor cells can be inducibly labeled with the fluorescent protein, Tomato, following tamoxifen administration. We investigated the effects of chronic fluoxetine on cell proliferation and nestin+ progenitor cells in periventricular areas in the medial hypothalamus and medial habenula, two brain areas involved in stress and anxiety responses.
Results: Our data provide the first in vivo evidence that fluoxetine promotes cell proliferation and neurogenesis and increases the mRNA levels of BDNF in the hypothalamus and habenula.
Conclusions: By identifying novel cellular targets of fluoxetine, our results may provide new insight into the mechanisms underlying antidepressant responses.
Keywords: antidepressant; habenula; hypothalamus; neurogenesis.
© The Author 2015. Published by Oxford University Press on behalf of CINP.
Figures
Figure 1.
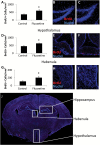
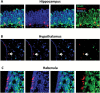
Fluoxetine leads to increased cell proliferation in the brain. (A) Quantification of BrdU incorporation data in the hippocampus of control and FLX-treated animals. Representative images from the hippocampus of control (B) and FLX-treated (C) mice are also shown. (D) Quantification of BrdU incorporation data in the hypothalamus of control and FLX-treated animals. Representative images from the hypothalamus of control (E) and FLX-treated (F) mice are also shown. (G) Quantification of BrdU incorporation data in the habenula of control and FLX-treated animals. Representative images from the hippocampus of control (H) and FLX-treated (I) mice are also shown. (J) A whole brain image with the hippocampus, habenula, and hypothalamus marked is shown for reference. BrdU is shown in red, nuclei are shown in blue. n = 10 control and 11 FLX for A–C; n = 15 per group for D–F; n = 10 per group for G–I. *p < 0.05 by _t_-test.
Figure 2.
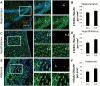
Fluoxetine does not affect the percentage of BrdU+ cells that become neurons. A representative image from the hippocampus (A), hypothalamus (C), and habenula (E) of a FLX-treated mouse is shown, and quantification is shown for the hippocampus (B), hypothalamus (D), and habenula (F). For all images, BrdU is shown in red, NeuN is shown in green, and nuclei are shown in blue. Arrowheads indicate double-positive cells. n = 9–11 per group.
Figure 3.
Fluoxetine does not affect the percentage of BrdU+ cells that become astrocytes. Representative images from the hippocampus (A), hypothalamus (C), and habenula (E) of a FLX-treated mouse are shown. Quantification of these results is presented for the hippocampus (B), hypothalamus (D), and habenula (F). For all images, BrdU is shown in red, GFAP is shown in green, and nuclei are shown in blue. n = 8–10 per group.
Figure 4.
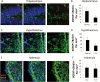
Fluoxetine leads to increased numbers of nestin+ cells in the brain. (A) Quantification of the number of Tomato+ cells in the hippocampus with representative images from control (B) and FLX-treated (C) animals. (D) Quantification of the number of Tomato+ cells in the hypothalamus, with representative images from control (E) and FLX-treated (F) animals. (G) Quantification of the number of Tomato+ cells in the habenula with representative images from control (H) and FLX-treated (I) animals. Tomato expression is shown in red, nuclei are shown in blue. n = 8 per group. *p < 0.05 by _t_-test.
Figure 5.
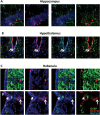
Neurogenesis from nestin+ precursors. Representative images of nestin-Tomato+/NeuN+ neurons in the hippocampus (A), hypothalamus (B), and (C) habenula. Tomato expression is shown in red, NeuN expression is shown in green, and nuclei are shown in blue. Arrows indicate double-positive cells.
Figure 6.
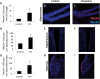
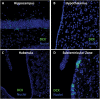
Doublecortin immunoreactivity in adult mouse brain. Representative images from the hippocampus (A), hypothalamus (B), habenula (C), and subventricular (D) zone of the lateral ventricle. Nuclei are shown in blue, and doublecortin (DCX) is shown in green.
Figure 7.
Gliogenesis from nestin+ precursors. Representative images of nestin-Tomato+ /GFAP+ glia in the hippocampus (A), hypothalamus (B), and habenula (C, D) in NCERT mice. Tomato expression is shown in red, NeuN expression is shown in green, and nuclei are shown in blue. C demonstrates the medial habenula itself, whereas D shows the region immediately dorsal to the medial habenula, the stria medullaris of the thalamus. Tomato expression is shown in red, GFAP expression is shown in green, and nuclei are shown in blue. Arrows indicate double-positive cells.
Figure 8.
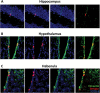
Vimentin expression in nestin-Tomato+ cells in NCERT mice. Representative images of nestin-Tomato+ cells (red) in the hippocampus (A), hypothalamus (B), and habenula (C) that stain positive with vimentin (green). Nuclei are shown in blue.
Figure 9.
BDNF and CREB mRNA expression in the hypothalamus and habenula following chronic FLX. Quantification of real-time PCR data reveals that chronic FLX leads to an increase in the expression of BDNF in the hypothalamus (A) and habenula (B), but not to an increase in CREB in these brain regions (C and D). *p < 0.05 by _t_-test. n = 7 per group.
Similar articles
- Requirement of AQP4 for antidepressive efficiency of fluoxetine: implication in adult hippocampal neurogenesis.
Kong H, Sha LL, Fan Y, Xiao M, Ding JH, Wu J, Hu G. Kong H, et al. Neuropsychopharmacology. 2009 Apr;34(5):1263-76. doi: 10.1038/npp.2008.185. Epub 2008 Oct 15. Neuropsychopharmacology. 2009. PMID: 18923397 - Protein kinase Mζ is involved in the modulatory effect of fluoxetine on hippocampal neurogenesis in vitro.
Wang YX, Zhang XR, Zhang ZJ, Li L, Xi GJ, Wu D, Wang YJ. Wang YX, et al. Int J Neuropsychopharmacol. 2014 Sep;17(9):1429-41. doi: 10.1017/S1461145714000364. Epub 2014 Mar 28. Int J Neuropsychopharmacol. 2014. PMID: 24679950 - Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression.
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. David DJ, et al. Neuron. 2009 May 28;62(4):479-93. doi: 10.1016/j.neuron.2009.04.017. Neuron. 2009. PMID: 19477151 Free PMC article. - Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise.
Micheli L, Ceccarelli M, D'Andrea G, Tirone F. Micheli L, et al. Brain Res Bull. 2018 Oct;143:181-193. doi: 10.1016/j.brainresbull.2018.09.002. Epub 2018 Sep 17. Brain Res Bull. 2018. PMID: 30236533 Review. - Promoting adult hippocampal neurogenesis: a novel strategy for antidepressant drug screening.
Yan HC, Cao X, Gao TM, Zhu XH. Yan HC, et al. Curr Med Chem. 2011;18(28):4359-67. doi: 10.2174/092986711797200471. Curr Med Chem. 2011. PMID: 21861813 Review.
Cited by
- Habenular volume changes after venlafaxine treatment in patients with major depression.
Etienne J, Boutigny A, David DJ, Deflesselle E, Gressier F, Becquemont L, Corruble E, Colle R. Etienne J, et al. Psychiatry Clin Neurosci. 2024 Aug;78(8):468-472. doi: 10.1111/pcn.13684. Epub 2024 Jun 12. Psychiatry Clin Neurosci. 2024. PMID: 38867362 Free PMC article. - Chronic Treatment with Serotonin Selective Reuptake Inhibitors Does Not Affect Regrowth of Serotonin Axons Following Amphetamine Injury in the Mouse Forebrain.
Janowitz HN, Linden DJ. Janowitz HN, et al. eNeuro. 2024 Feb 14;11(2):ENEURO.0444-22.2023. doi: 10.1523/ENEURO.0444-22.2023. Print 2024 Feb. eNeuro. 2024. PMID: 38355299 Free PMC article. - Central 5-HTergic hyperactivity induces myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-like pathophysiology.
Lee JS, Kang JY, Park SY, Hwang SJ, Bae SJ, Son CG. Lee JS, et al. J Transl Med. 2024 Jan 8;22(1):34. doi: 10.1186/s12967-023-04808-x. J Transl Med. 2024. PMID: 38191373 Free PMC article. - Dietary Astaxanthin: A Promising Antioxidant and Anti-Inflammatory Agent for Brain Aging and Adult Neurogenesis.
Medoro A, Davinelli S, Milella L, Willcox BJ, Allsopp RC, Scapagnini G, Willcox DC. Medoro A, et al. Mar Drugs. 2023 Dec 16;21(12):643. doi: 10.3390/md21120643. Mar Drugs. 2023. PMID: 38132964 Free PMC article. Review. - Serotonergic mediation of the brain-wide neurogenesis: Region-dependent and receptor-type specific roles on neurogenic cellular transformation.
Higuchi Y, Arakawa H. Higuchi Y, et al. Curr Res Neurobiol. 2023 Jul 28;5:100102. doi: 10.1016/j.crneur.2023.100102. eCollection 2023. Curr Res Neurobiol. 2023. PMID: 37638344 Free PMC article. Review.
References
- Agetsuma M, Aizawa H, Aoki T, Nakayama R, Takahoko M, Goto M, Sassa T, Amo R, Shiraki T, Kawakami K, Hosoya T, Higashijima S, Okamoto H. (2010). The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci 13:1354–1356. - PubMed
- Anacker C, Pariante CM. (2012). Can adult neurogenesis buffer stress responses and depressive behaviour? Mol Psychiatry 17:9–10. - PubMed
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. (2009). The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14:764–773, 739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 MH060451/MH/NIMH NIH HHS/United States
- MH60451/MH/NIMH NIH HHS/United States
- F32- MH093092/MH/NIMH NIH HHS/United States
- R01 MH079201/MH/NIMH NIH HHS/United States
- MH79201/MH/NIMH NIH HHS/United States
- F32 MH093092/MH/NIMH NIH HHS/United States
- MH79201-03S1/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources