Alteration of lysosome fusion and low-grade inflammation mediated by super-low-dose endotoxin - PubMed (original) (raw)
Alteration of lysosome fusion and low-grade inflammation mediated by super-low-dose endotoxin
Bianca Baker et al. J Biol Chem. 2015.
Abstract
Subclinical super-low-dose endotoxin LPS is a risk factor for the establishment of low-grade inflammation during the pathogenesis and progression of chronic diseases. However, the underlying mechanisms are not well understood. At the cellular level, a disruption of lysosome fusion with endosomes or autophagosomes may contribute to the potentiation of low-grade inflammation. In this study, we identified that subclinical super-low-dose endotoxin LPS can potently inhibit the process of endosome acidification and lysosome fusion with endosomes or autophagosomes in primary macrophages. Super-low-dose LPS induced the inhibitory phosphorylation of VPS34, thus leading to the disruption of endosome-lysosome fusion. This effect may depend upon the clearance and relocation of Tollip in macrophages by super-low-dose LPS. Consistent with this notion, Tollip-deficient macrophages had constitutively elevated levels of VPS34 inhibitory phosphorylation and constitutive disruption of endosome-lysosome fusion. By employing a skin excision wound-healing model, we observed that Tollip-deficient mice had significantly elevated levels of cell stress and reduced wound repair. This study reveals a novel mechanism responsible for the modulation of endosome-lysosome fusion and low-grade inflammation in innate macrophages.
Keywords: Cell Signaling; Endosome-Lysosome Fusion; Innate Immunity; Lipopolysaccharide (LPS); Low-grade Inflammation; Lysosome; Macrophage; Macrophages; Super-low-dose Endotoxin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
FIGURE 1.
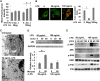
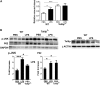
Super-low-dose LPS suppresses lysosome fusion. A, WT macrophages treated with either PBS or LPS (50 pg/ml or 100 ng/ml) were subjected to endosome acidification assays by incorporating pH-sensitive dextran double-labeled with FITC and tetramethylrhodamine. Flow cytometry analyses were performed to monitor the increase in fluorescence intensity, which indicates a decrease in endosomal pH. *, p < 0.05 compared with untreated controls. B, WT macrophages treated with either PBS or 50 pg/ml LPS were subjected to endosome-lysosome fusion assays by sequential incorporation of Alexa Fluor 546-labeled dextran and FITC-labeled zymosan BioParticles. Fluorescence images were obtained with the Zeiss LSM 510 laser-scanning confocal microscope to observe isolated lysosomes (red), endosomes (green), and fused endolysosomes (orange). The percentage of cells with fused endolysosomes is represented in the graph. *, p < 0.05 compared with untreated controls. C, WT macrophages treated with PBS or 50 pg/ml LPS for 2 h were subjected to analyses by transmission electron microscopy. Dark electron-dense lysosome structures can be seen in the samples challenged with 50 pg/ml LPS. D, WT macrophages were treated with either 50 pg/ml or 100 ng/ml LPS for the indicated times. The levels of p62 and GAPDH controls were determined by Western blot analyses. Data represent three experiments. *, p < 0.05 compared with untreated controls. E, WT macrophages were treated with either 50 pg/ml or 100 ng/ml LPS for the indicated times. The levels of phosphorylated (p) and total JNK, ERK, and p38 were determined by Western blotting. Data represent three experiments.
FIGURE 2.
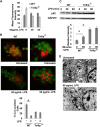
Tollip facilitates lysosome fusion. A, WT and Tollip-deficient macrophages were treated with 50 pg/ml LPS for the indicated times. Endosome acidification assays were performed, followed by flow cytometry analysis. B, WT and Tollip-deficient macrophages were treated with 50 pg/ml LPS. Assays that measure lysosome fusion with endosome were performed, followed by confocal fluorescence microscopy analysis. The percentage of cells with fused endolysosomes are represented in the graph. *, p < 0.05 compared with untreated controls. C, WT and Tollip-deficient macrophages were treated with 50 pg/ml for the indicated times. The expression levels of p62 and GAPDH controls were determined by Western blot analyses. Data represent three experiments. *, p < 0.05 compared with untreated controls. D, Tollip-deficient macrophages treated with PBS or 50 pg/ml LPS for 2 h were subjected to transmission electron microscopy analyses. Dark electron-dense lysosome structures can be seen in the samples challenged with or without LPS challenge.
FIGURE 3.
Super-low-dose LPS suppresses AMPK and VPS34. WT and Tollip-deficient macrophages were treated with 50 pg/ml LPS for the indicated times. The levels of phosphorylated (p) and total AMPK (A) and VPS34 (B) were determined by Western blot analyses. The relative levels of phosphorylated AMPK and VPS34 were quantified from three experiments. *, p < 0.05 compared with untreated controls.
FIGURE 4.
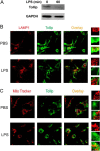
Clearance and removal of Tollip from late endosomes/lysosomes by super-low-dose LPS. A, WT macrophages were treated with PBS or 50 pg/ml LPS for 1 h. Total cell lysates were harvested, and equal amounts of protein lysates were resolved by SDS-PAGE. The levels of Tollip and control GAPDH were visualized by Western blotting. WT macrophages were treated with 50 pg/ml LPS for either 1 h (B) or 24 h (C). Cells were stained with goat anti-mouse Tollip antibody, followed by Alexa Fluor 488-labeled rabbit anti-goat IgG together with either Cy3-conjugated LAMP1-specific antibody (B) or MitoTracker Red (C). The intracellular distribution of Tollip in late endosomes/lysosomes or mitochondrial compartments was visualized by confocal fluorescence microscopy.
FIGURE 5.
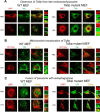
Cells with mutant Tollip constitutively localized at late endosomes/lysosomes exhibit lysosome fusion in the presence of super-low-dose LPS. WT and Tollip M240A/F241A mutant MEF cells were treated with PBS or 50 pg/ml LPS for 24 h. A and B, the intracellular distribution of Tollip in late endosomes/lysosomes or mitochondrial compartments was visualized by confocal fluorescence microscopy. Cells were stained with goat anti-mouse Tollip antibody, followed by Alexa Fluor 488-labeled rabbit anti-goat IgG together with either Cy3-conjugated LAMP1-specific antibody (A) or MitoTracker Red (B). C, fusion of lysosomes with autophagosomes was visualized by staining autophagosomes with Alexa Fluor 488-labeled LC3 (LCIII) antibody and Cy3-conjugated LAMP1-specific antibody.
FIGURE 6.
Impaired wound repair and increased inflammatory monocytes in wound tissues in Tollip-deficient mice. A, WT and Tollip-deficient mice were injected intraperitoneally with either PBS or 100 pg/mouse LPS every 3 days for 10 days. On day 10, the lower backs of the mice were punctured with 4-mm full skin-depth wounds and monitored daily for wound size. The ratios of the unhealed wound sizes to the original wounds are plotted (n > 7). *, p < 0.05; ***, p < 0.001. B, total protein lysates were harvested from wound tissues from WT and Tollip-deficient mice. The levels of phospho-JNK (p-JNK), p62, and Tollip were determined by Western blotting. Representative blots are shown. The relative intensities of p62 and phospho-JNK (12 WT samples and six Tollip-deficient samples) were adjusted with GAPDH loading controls and are plotted. *, p < 0.05; ***, p < 0.001.
FIGURE 7.
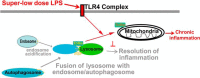
Schematic diagram of the role and regulation of Tollip in innate macrophages challenged with super-low-dose endotoxin.
Similar articles
- Low-grade inflammatory polarization of monocytes impairs wound healing.
Yuan R, Geng S, Chen K, Diao N, Chu HW, Li L. Yuan R, et al. J Pathol. 2016 Mar;238(4):571-83. doi: 10.1002/path.4680. Epub 2016 Jan 25. J Pathol. 2016. PMID: 26690561 Free PMC article. - Molecular and cellular mechanisms responsible for cellular stress and low-grade inflammation induced by a super-low dose of endotoxin.
Baker B, Maitra U, Geng S, Li L. Baker B, et al. J Biol Chem. 2014 Jun 6;289(23):16262-9. doi: 10.1074/jbc.M114.569210. Epub 2014 Apr 22. J Biol Chem. 2014. PMID: 24759105 Free PMC article. - Toll-interacting protein deficiency promotes neurodegeneration via impeding autophagy completion in high-fat diet-fed ApoE-/- mouse model.
Chen K, Yuan R, Geng S, Zhang Y, Ran T, Kowalski E, Liu J, Li L. Chen K, et al. Brain Behav Immun. 2017 Jan;59:200-210. doi: 10.1016/j.bbi.2016.10.002. Epub 2016 Oct 5. Brain Behav Immun. 2017. PMID: 27720815 Free PMC article. - How do cells optimize luminal environments of endosomes/lysosomes for efficient inflammatory responses?
Kobayashi T, Tanaka T, Toyama-Sorimachi N. Kobayashi T, et al. J Biochem. 2013 Dec;154(6):491-9. doi: 10.1093/jb/mvt099. Epub 2013 Oct 31. J Biochem. 2013. PMID: 24178399 Review. - Contribution of inflammatory pathways to Fabry disease pathogenesis.
Rozenfeld P, Feriozzi S. Rozenfeld P, et al. Mol Genet Metab. 2017 Nov;122(3):19-27. doi: 10.1016/j.ymgme.2017.09.004. Epub 2017 Sep 13. Mol Genet Metab. 2017. PMID: 28947349 Review.
Cited by
- Macrophage-Specific Hypoxia-Inducible Factor-1α Contributes to Impaired Autophagic Flux in Nonalcoholic Steatohepatitis.
Wang X, de Carvalho Ribeiro M, Iracheta-Vellve A, Lowe P, Ambade A, Satishchandran A, Bukong T, Catalano D, Kodys K, Szabo G. Wang X, et al. Hepatology. 2019 Feb;69(2):545-563. doi: 10.1002/hep.30215. Epub 2019 Jan 4. Hepatology. 2019. PMID: 30102772 Free PMC article. - TFEB Dependent Autophagy-Lysosomal Pathway: An Emerging Pharmacological Target in Sepsis.
Liu X, Zheng X, Lu Y, Chen Q, Zheng J, Zhou H. Liu X, et al. Front Pharmacol. 2021 Nov 26;12:794298. doi: 10.3389/fphar.2021.794298. eCollection 2021. Front Pharmacol. 2021. PMID: 34899355 Free PMC article. Review. - Toll-Interacting Protein in Resolving and Non-Resolving Inflammation.
Kowalski EJA, Li L. Kowalski EJA, et al. Front Immunol. 2017 May 5;8:511. doi: 10.3389/fimmu.2017.00511. eCollection 2017. Front Immunol. 2017. PMID: 28529512 Free PMC article. Review. - Toll interacting protein protects bronchial epithelial cells from bleomycin-induced apoptosis.
Li X, Kim SE, Chen TY, Wang J, Yang X, Tabib T, Tan J, Guo B, Fung S, Zhao J, Sembrat J, Rojas M, Shiva S, Lafyatis R, St Croix C, Alder JK, Di YP, Kass DJ, Zhang Y. Li X, et al. FASEB J. 2020 Aug;34(8):9884-9898. doi: 10.1096/fj.201902636RR. Epub 2020 Jun 28. FASEB J. 2020. PMID: 32596871 Free PMC article. - Role of gut microbiota in the modulation of atherosclerosis-associated immune response.
Chistiakov DA, Bobryshev YV, Kozarov E, Sobenin IA, Orekhov AN. Chistiakov DA, et al. Front Microbiol. 2015 Jun 30;6:671. doi: 10.3389/fmicb.2015.00671. eCollection 2015. Front Microbiol. 2015. PMID: 26175728 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- AI106287/AI/NIAID NIH HHS/United States
- R01 HL115835/HL/NHLBI NIH HHS/United States
- R01 AI106287/AI/NIAID NIH HHS/United States
- R01HL115835/HL/NHLBI NIH HHS/United States
- R25 GM072767/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases