R-loops highlight the nucleus in ALS - PubMed (original) (raw)
Review
R-loops highlight the nucleus in ALS
Jayesh S Salvi et al. Nucleus. 2015.
Abstract
Amyotrophic lateral sclerosis (ALS) is a severely debilitating neurodegenerative disease linked to mutations in various genes implicated in cytoplasmic RNA metabolism. Recent studies from genetic models have also helped reveal connections between various ALS-linked factors and RNA-DNA hybrid (R-loop) regulation. Here, we examine how such hybrid-regulatory processes are pointing to a key role for the nucleus in ALS. We also present a potential molecular mechanism in which hybrids may represent at least one of the long sought after missing links between different ALS genes. Our opinion is that RNA-DNA hybrids will play a key role in deciphering ALS and other human diseases.
Keywords: Amyotrophic lateral sclerosis (ALS); Ataxin2; C9ORF72; FUS; R-loop; RNA-DNA hybrid; SOD1; Senataxin; TDP43; genome instability; stress granules.
Figures
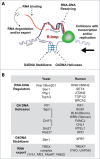
Figure 1.
R-loop regulatory factors. (A) RNA modulatory processes involving RNA binding, degradation, and export cooperate with RNA-DNA hybrid resolving/suppressing factors and G4DNA helicases to suppress R-loop accumulation. On the other hand, G4DNA stabilizing factors can promote R-loop accumulation. R-loop accumulation can lead to genome-destabilizing collisions with transcription and/or replication machinery. (B) Examples of yeast and human R-loop regulators that may or may not be linked to ALS pathobiology.
Figure 2.
Putative feedback loop between R-loops and stress granules may connect various ALS genes at the molecular level. Mutations (μ) in ALS-linked factors can abrogate their ability to suppress R-loop formation and/or lead to their aberrant sequestration in RNA-processing foci such as cytoplasmic stress granules. Increased R-loop accumulation in turn leads to genomic instability and aberrant transcript accumulations, which in turn further promotes stress granule formation and the cycle continues. Motor neurons are predicted to be particularly sensitive to such a deleterious and vicious cycle.
Similar articles
- ALS-Related Mutant FUS Protein Is Mislocalized to Cytoplasm and Is Recruited into Stress Granules of Fibroblasts from Asymptomatic FUS P525L Mutation Carriers.
Lo Bello M, Di Fini F, Notaro A, Spataro R, Conforti FL, La Bella V. Lo Bello M, et al. Neurodegener Dis. 2017;17(6):292-303. doi: 10.1159/000480085. Epub 2017 Oct 17. Neurodegener Dis. 2017. PMID: 29035885 - Mutations in the 3' untranslated region of FUS causing FUS overexpression are associated with amyotrophic lateral sclerosis.
Sabatelli M, Moncada A, Conte A, Lattante S, Marangi G, Luigetti M, Lucchini M, Mirabella M, Romano A, Del Grande A, Bisogni G, Doronzio PN, Rossini PM, Zollino M. Sabatelli M, et al. Hum Mol Genet. 2013 Dec 1;22(23):4748-55. doi: 10.1093/hmg/ddt328. Epub 2013 Jul 11. Hum Mol Genet. 2013. PMID: 23847048 - Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis.
Kwiatkowski TJ Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH Jr. Kwiatkowski TJ Jr, et al. Science. 2009 Feb 27;323(5918):1205-8. doi: 10.1126/science.1166066. Science. 2009. PMID: 19251627 - The evidence for altered RNA metabolism in amyotrophic lateral sclerosis (ALS).
Strong MJ. Strong MJ. J Neurol Sci. 2010 Jan 15;288(1-2):1-12. doi: 10.1016/j.jns.2009.09.029. Epub 2009 Oct 18. J Neurol Sci. 2010. PMID: 19840884 Review. - RNA-binding proteins and RNA metabolism: a new scenario in the pathogenesis of Amyotrophic lateral sclerosis.
Colombrita C, Onesto E, Tiloca C, Ticozzi N, Silani V, Ratti A. Colombrita C, et al. Arch Ital Biol. 2011 Mar;149(1):83-99. doi: 10.4449/aib.v149i1.1261. Arch Ital Biol. 2011. PMID: 21412718 Review.
Cited by
- Intersection of calorie restriction and magnesium in the suppression of genome-destabilizing RNA-DNA hybrids.
Abraham KJ, Chan JN, Salvi JS, Ho B, Hall A, Vidya E, Guo R, Killackey SA, Liu N, Lee JE, Brown GW, Mekhail K. Abraham KJ, et al. Nucleic Acids Res. 2016 Oct 14;44(18):8870-8884. doi: 10.1093/nar/gkw752. Epub 2016 Aug 29. Nucleic Acids Res. 2016. PMID: 27574117 Free PMC article. - TDP-43/FUS in motor neuron disease: Complexity and challenges.
Guerrero EN, Wang H, Mitra J, Hegde PM, Stowell SE, Liachko NF, Kraemer BC, Garruto RM, Rao KS, Hegde ML. Guerrero EN, et al. Prog Neurobiol. 2016 Oct-Nov;145-146:78-97. doi: 10.1016/j.pneurobio.2016.09.004. Epub 2016 Sep 28. Prog Neurobiol. 2016. PMID: 27693252 Free PMC article. Review. - NUSAP1 Binds ILF2 to Modulate R-Loop Accumulation and DNA Damage in Prostate Cancer.
Chiu CL, Li CG, Verschueren E, Wen RM, Zhang D, Gordon CA, Zhao H, Giaccia AJ, Brooks JD. Chiu CL, et al. Int J Mol Sci. 2023 Mar 26;24(7):6258. doi: 10.3390/ijms24076258. Int J Mol Sci. 2023. PMID: 37047232 Free PMC article. - Chronic oxidative damage together with genome repair deficiency in the neurons is a double whammy for neurodegeneration: Is damage response signaling a potential therapeutic target?
Wang H, Dharmalingam P, Vasquez V, Mitra J, Boldogh I, Rao KS, Kent TA, Mitra S, Hegde ML. Wang H, et al. Mech Ageing Dev. 2017 Jan;161(Pt A):163-176. doi: 10.1016/j.mad.2016.09.005. Epub 2016 Sep 20. Mech Ageing Dev. 2017. PMID: 27663141 Free PMC article. Review. - Tissue-Specific, Genome-wide Mapping of R-loops in Drosophila Using MapR.
Jauregui-Lozano J, Cottingham K, Hall H. Jauregui-Lozano J, et al. Bio Protoc. 2022 Sep 20;12(18):e4516. doi: 10.21769/BioProtoc.4516. eCollection 2022 Sep 20. Bio Protoc. 2022. PMID: 36248608 Free PMC article.
References
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med 2001; 344:1688-700; PMID:11386269; http://dx.doi.org/10.1056/NEJM200105313442207 - DOI - PubMed
- Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993; 364:362; PMID:8332197 - PubMed
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, et al. . TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008; 319:1668-72; PMID:18309045; http://dx.doi.org/10.1126/science.1154584 - DOI - PMC - PubMed
- Rutherford NJ, Zhang YJ, Baker M, Gass JM, Finch NA, Xu YF, Stewart H, Kelley BJ, Kuntz K, Crook RJ, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. . Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet 2008; 4:e1000193; PMID:18802454; http://dx.doi.org/10.1371/journal.pgen.1000193 - DOI - PMC - PubMed
- Kwiatkowski TJ, Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. . Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009; 323:1205-8; PMID:19251627; http://dx.doi.org/10.1126/science.1166066 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous