Comparative diffusion tractography of corticostriatal motor pathways reveals differences between humans and macaques - PubMed (original) (raw)
Comparative Study
. 2015 Apr 1;113(7):2164-72.
doi: 10.1152/jn.00569.2014. Epub 2015 Jan 14.
Affiliations
- PMID: 25589589
- PMCID: PMC4416585
- DOI: 10.1152/jn.00569.2014
Comparative Study
Comparative diffusion tractography of corticostriatal motor pathways reveals differences between humans and macaques
S F W Neggers et al. J Neurophysiol. 2015.
Abstract
The primate corticobasal ganglia circuits are understood to be segregated into parallel anatomically and functionally distinct loops. Anatomical and physiological studies in macaque monkeys are summarized as showing that an oculomotor loop begins with projections from the frontal eye fields (FEF) to the caudate nucleus, and a motor loop begins with projections from the primary motor cortex (M1) to the putamen. However, recent functional and structural neuroimaging studies of the human corticostriatal system report evidence inconsistent with this organization. To obtain conclusive evidence, we directly compared the pattern of connectivity between cortical motor areas and the striatum in humans and macaques in vivo using probabilistic diffusion tractography. In macaques we found that FEF is connected with the head of the caudate and anterior putamen, and M1 is connected with more posterior sections of the caudate and putamen, corroborating neuroanatomical tract tracing findings. However, in humans FEF and M1 are connected to largely overlapping portions of posterior putamen and only a small portion of the caudate. These results demonstrate that the corticobasal connectivity for the oculomotor and primary motor loop is not entirely segregated for primates at a macroscopic level and that the description of the anatomical connectivity of corticostriatal motor systems in humans does not parallel that of macaques, perhaps because of an expansion of prefrontal projections to striatum in humans.
Keywords: DTI; comparative anatomy; human; macaque; oculomotor.
Copyright © 2015 the American Physiological Society.
Figures
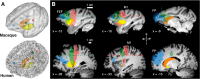
Fig. 1.
Cortical seeds, striatal targets, waypoint masks, and corticostriatal streamlines in macaques and humans. Seed regions were defined as spheres with a 2-mm (macaques) or 4-mm (humans) radius. A: semitransparent 3-dimensional rendering from the T1-weighted image for macaques (top) and humans (bottom) with embedded caudate (orange) and putamen (yellow) and cortical seed regions: frontal eye fields (FEF) (green), M1 (red), and frontal pole (FP; blue). B: sagittal slices for macaques (top) and humans (bottom) with overlaid cortical seed regions (FEF, left columns), M1 (middle columns), and frontal pole (right columns), the group-averaged fiber streamlines from these regions (transparent corresponding colors), and the striatal regions caudate (orange) and putamen (yellow). Dashed lines show waypoint mask planes. Slice coordinates are in template space [Montreal Neurological Institute (MNI) space for humans, 112RM-SL space for macaques]. A, anterior; I, inferior; P, posterior; S, superior.
Fig. 2.
Classification maps of the probability a cortical seed was connected to the putamen and caudate nucleus, overlaid on 2-dimensional slices through the average normalized T1-weighted scan at the level of the striatum. The opacity of the overlay indicates the probability of connectivity (transparent voxels have a probability equal to 0; opaque voxels have a probability equal to 1).
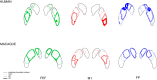
Fig. 3.
Classification analysis of corticostriatal fibers in macaque and human. A: corticostriatal terminations in macaque (top) and human (bottom), pooled across hemispheres displayed on left-right (L-R) side views of caudate and putamen. Opaque colors indicate fiber termination zones based on group probability maps thresholded at P > 0.2. Arrows show orientation. B: distribution of corticostriatal connectivity and striatal volumes along the anterior-posterior (A-P) axis. Line charts show probability densities of total striatal volume along the A-P axis for macaque (dashed lines) and human (solid lines). The total area under the line charts represents the total striatal volume. Area charts show probability densities of corticostriatal connectivity along the A-P axis for macaque (vertically striped areas) and human (horizontally striped areas). For comparison across species and striatal regions, volume and fiber termination densities were normalized, so that the area under the line charts is equal to 1.
Fig. 4.
Connectivity index connectivity index (IC) as a function of group level probability threshold for data from M1 (red), FEF (green), and frontal pole (blue) from macaques (triangles) and humans (circles). This threshold reflects the number of subjects (humans or macaques) out of 7 for which a voxel was tagged at the single subject level that was needed to accept a voxel as being connected to the respective cortical seed at the group level. The size of each data point is proportional to the number of voxels on which the labeling index was computed. Data points for thresholds yielding connectivity <5% of the total striatal volume are not shown.
Fig. 5.
The classification analysis was repeated for 3 different minimum curvature thresholds used during probabilistic tractography: 120° allowing only relatively straight streamlines, 80° (the default value used in all the other analyses in this study), and 40° allowing very sharp bends in assessed streamlines. Top: outlines of the putamen and caudate as thin black lines projected on an axial slice through the striatum for humans and in colored lines with increasing thickness (reflecting decreasing minimal curvature) the outlines of the zones connected to the cortical seeds from left to right for the FEF (in green), M1 (in red), and frontal pole (in blue). Bottom: same data for macaques.
Similar articles
- Distributed but convergent ordering of corticostriatal projections: analysis of the frontal eye field and the supplementary eye field in the macaque monkey.
Parthasarathy HB, Schall JD, Graybiel AM. Parthasarathy HB, et al. J Neurosci. 1992 Nov;12(11):4468-88. doi: 10.1523/JNEUROSCI.12-11-04468.1992. J Neurosci. 1992. PMID: 1279139 Free PMC article. - Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study.
Leh SE, Ptito A, Chakravarty MM, Strafella AP. Leh SE, et al. Neurosci Lett. 2007 May 29;419(2):113-8. doi: 10.1016/j.neulet.2007.04.049. Epub 2007 May 4. Neurosci Lett. 2007. PMID: 17485168 Free PMC article. - Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum.
Jarbo K, Verstynen TD. Jarbo K, et al. J Neurosci. 2015 Mar 4;35(9):3865-78. doi: 10.1523/JNEUROSCI.2636-14.2015. J Neurosci. 2015. PMID: 25740516 Free PMC article. - Striatal tissue transplantation in non-human primates.
Kendall AL, Hantraye P, Palfi S. Kendall AL, et al. Prog Brain Res. 2000;127:381-404. doi: 10.1016/s0079-6123(00)27018-0. Prog Brain Res. 2000. PMID: 11142037 Review. - [Cortico-basal ganglia circuits--parallel closed loops and convergent/divergent connections].
Miyachi S. Miyachi S. Brain Nerve. 2009 Apr;61(4):351-9. Brain Nerve. 2009. PMID: 19378804 Review. Japanese.
Cited by
- Parallel basal ganglia circuits for voluntary and automatic behaviour to reach rewards.
Kim HF, Hikosaka O. Kim HF, et al. Brain. 2015 Jul;138(Pt 7):1776-800. doi: 10.1093/brain/awv134. Epub 2015 May 16. Brain. 2015. PMID: 25981958 Free PMC article. Review. - Hypoxia-Ischemia and Hypothermia Independently and Interactively Affect Neuronal Pathology in Neonatal Piglets with Short-Term Recovery.
O'Brien CE, Santos PT, Kulikowicz E, Reyes M, Koehler RC, Martin LJ, Lee JK. O'Brien CE, et al. Dev Neurosci. 2019;41(1-2):17-33. doi: 10.1159/000496602. Epub 2019 May 20. Dev Neurosci. 2019. PMID: 31108487 Free PMC article. - Combining diffusion magnetic resonance tractography with stereology highlights increased cross-cortical integration in primates.
Charvet CJ, Hof PR, Raghanti MA, Van Der Kouwe AJ, Sherwood CC, Takahashi E. Charvet CJ, et al. J Comp Neurol. 2017 Apr 1;525(5):1075-1093. doi: 10.1002/cne.24115. Epub 2016 Nov 22. J Comp Neurol. 2017. PMID: 27615357 Free PMC article. - A supramodal role of the basal ganglia in memory and motor inhibition: Meta-analytic evidence.
Guo Y, Schmitz TW, Mur M, Ferreira CS, Anderson MC. Guo Y, et al. Neuropsychologia. 2018 Jan 8;108:117-134. doi: 10.1016/j.neuropsychologia.2017.11.033. Epub 2017 Dec 1. Neuropsychologia. 2018. PMID: 29199109 Free PMC article. - Negative childhood experiences alter a prefrontal-insular-motor cortical network in healthy adults: A preliminary multimodal rsfMRI-fMRI-MRS-dMRI study.
Duncan NW, Hayes DJ, Wiebking C, Tiret B, Pietruska K, Chen DQ, Rainville P, Marjańska M, Ayad O, Doyon J, Hodaie M, Northoff G. Duncan NW, et al. Hum Brain Mapp. 2015 Nov;36(11):4622-37. doi: 10.1002/hbm.22941. Epub 2015 Aug 19. Hum Brain Mapp. 2015. PMID: 26287448 Free PMC article.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381, 1986. - PubMed
- Amiez C, Petrides M. Anatomical organization of the eye fields in the human and non-human primate frontal cortex. Prog Neurobiol 89: 220–320, 2009. - PubMed
- Andersson JL, Skare S. A model-based method for retrospective correction of geometric distortions in diffusion-weighted EPI. Neuroimage 16: 177–199, 2002. - PubMed
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage 26: 839–851, 2005. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources