Screen identifies bromodomain protein ZMYND8 in chromatin recognition of transcription-associated DNA damage that promotes homologous recombination - PubMed (original) (raw)
. 2015 Jan 15;29(2):197-211.
doi: 10.1101/gad.252189.114.
Li-Ya Chiu 1, Ben Cox 1, François Aymard 2, Thomas Clouaire 2, Justin W Leung 1, Michael Cammarata 3, Mercedes Perez 1, Poonam Agarwal 1, Jennifer S Brodbelt 3, Gaëlle Legube 2, Kyle M Miller 4
Affiliations
- PMID: 25593309
- PMCID: PMC4298138
- DOI: 10.1101/gad.252189.114
Screen identifies bromodomain protein ZMYND8 in chromatin recognition of transcription-associated DNA damage that promotes homologous recombination
Fade Gong et al. Genes Dev. 2015.
Abstract
How chromatin shapes pathways that promote genome-epigenome integrity in response to DNA damage is an issue of crucial importance. We report that human bromodomain (BRD)-containing proteins, the primary "readers" of acetylated chromatin, are vital for the DNA damage response (DDR). We discovered that more than one-third of all human BRD proteins change localization in response to DNA damage. We identified ZMYND8 (zinc finger and MYND [myeloid, Nervy, and DEAF-1] domain containing 8) as a novel DDR factor that recruits the nucleosome remodeling and histone deacetylation (NuRD) complex to damaged chromatin. Our data define a transcription-associated DDR pathway mediated by ZMYND8 and the NuRD complex that targets DNA damage, including when it occurs within transcriptionally active chromatin, to repress transcription and promote repair by homologous recombination. Thus, our data identify human BRD proteins as key chromatin modulators of the DDR and provide novel insights into how DNA damage within actively transcribed regions requires chromatin-binding proteins to orchestrate the appropriate response in concordance with the damage-associated chromatin context.
Keywords: DNA damage; ZMYND8; bromodomain; chromatin; histone acetylation.
© 2015 Gong et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
Figure 1.
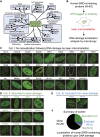
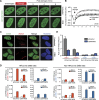
Comprehensive screening of human BRD protein relocalization following DNA damage. (A) Human BRD protein family, organized by known functions and the classifications from Filippakopoulos et al. (2012). (B) DNA damage relocalization screen for BRD proteins. (C_–_E) Screening results were sorted into three categories. (C) Category I: no relocalization following DNA damage. (D) Category II: recruited to DNA damage. (E) Category III: excluded from DNA damage. (F) Summary of screen results. (NA) Not analyzed.
Figure 2.
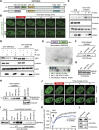
TIP60-mediated H4 acetylation recruits ZMYND8 to damaged chromatin through BRD recognition. (A) ZMYND8 domain organization, including the PHD, BRD, PWWP domain, and MYND domain. (B) Laser damage recruitment of GFP-tagged full-length (FL) but not BRD-deleted (ΔBRD) ZMYND8. The dotted line indicates the laser path. (C) Endogenous ZMYND8 accumulates on chromatin following DNA damage by IR. Cells treated and analyzed as in D with the indicated antibodies. (D) Chromatin association of GFP-ZMYND8 following IR is BRD-dependent. Whole-cell extract (WCE) and chromatin (C) fractions obtained from untreated or IR-treated cells and analyzed by Western blotting. (E) Recombinant ZMYND8 PHD–BRD (GST-PB-His) binds H4Ac. Binding assays with the histone peptide arrays were performed as described in the Materials and Methods. Highly bound peptides are indicated. (Blue box) Modified H3K36 peptides. (F) Validation of the H4Ac interaction from E by peptide pull-down assay. Pull-down of recombinant ZMYND8 by the indicated peptides. (G) Coomassie staining of the loading control for H4 peptides. (H) Endogenous ZMYND8 from HeLa nuclear extracts binds H4Ac peptides. Extracts from siRNA-treated cells serves as control for ZMYND8 antibody specificity. β-Tubulin acted as a loading control. (I) GFP-ZMYND8 H4Ac binding requires the BRD. Experiments performed as in H using HEK293T cell extracts. A single N248A mutation within a conserved BRD acetyl-lysine-binding site reduces H4Ac interactions. (J) TIP60 depletion impairs ZMYND8 damage associations. U2OS cells stably expressing GFP-ZMYND8 and treated with siControl or siTIP60 were damaged within the dotted circles and imaged by live-cell microscopy. (K) Quantification of J. The difference in average fluorescence intensity of GFP-ZMYND8 in damaged versus undamaged regions is plotted at each time point. Error bars indicate SEM; n > 10. (L) TIP60 depletion reduces H4Ac. Extracts from siControl and siTIP60 cells were analyzed by Western blotting with the indicated antibodies. (H4 tetra-Ac) Acetylation of H4 at K5, K8, K12, and K16.
Figure 3.
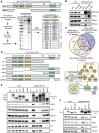
ZMYND8 interacts with chromatin and chromatin-modifying complexes NuRD and BHC. (A) Identification of ZMYND8-interacting factors by MS. Purification scheme of full-length (FL) and PHD–BRD–PWWP-deleted (ΔPBP) SFB-tagged ZMYND8. (SBP) Streptavidin-binding peptide. The table represents ZMYND8 interactors identified in mass spectrum from the indicated experiments. (B) Endogenous ZMYND8 interacts with CHD4, a core component of the NuRD complex. Western blotting analysis of reciprocal co-IPs with the indicated antibodies from HEK293T cells. (C) ZMYND8 MS data. Overlap of full-length (n = 2) and ΔPBP ZMYND8 interactors. (Bottom) Individual interactions based on literature and our MS results. (D_–_F) The BRD of ZMYND8 interacts with chromatin, and the MYND domain interacts with the NuRD and BHC complexes. (D) Full-length and mutant SFB-ZMYND8 constructs. (E,F) Mapping ZMYND8 interaction domains with interactors. ZMYND8 constructs were transfected into HEK293T and analyzed by co-IP. Full-length and mutant SFB-ZMYND8 were purified using streptavidin beads. (input) Whole-cell extracts. Purified complexes and input were analyzed by Western blotting.
Figure 4.
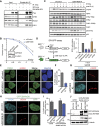
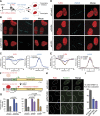
ZMYND8 participates in the DDR and recruits CHD4 to damaged chromatin. (A) ZMYND8 interacts with CHD4 upon IR treatment. Experiments were performed as in Figure 3E with IR. (B) Clonogenic assays reveal hypersensitivity of ZMYND8-depleted cells to IR. U2OS cells were treated with control or ZMYND8 siRNAs and damaged with various doses of IR. Graphs are mean ± SEM; n = 2. (C) ZMYND8-depleted cells are defective in DNA damage signaling. siControl and siZMYND8 U2OS cells were IR-treated and analyzed at the indicated time points by Western blotting. Several phosphorylated DNA damage markers were analyzed with unmodified antibodies acting as loading controls. (D) Cells depleted of ZMYND8 and CHD4, but not LSD1, are defective in HR. DR-GFP reporter assays were performed after depletion of the indicated proteins by siRNAs. Depletion of CtIP was used as a positive control. Error bars indicate SEM; n = 3. (E_–_G) Recruitment of CHD4 to laser damage requires ZMYND8. siControl and siZMYND8 U2OS cells were laser-damaged, and endogenous CHD4 accumulation was analyzed by immunofluorescence. (F) Quantification of E. Data were obtained from >50 cells from three independent experiments. Error bars indicate mean ± SEM. (G) The same results obtained as in E using an independent siRNA targeting the 3′ untranslated region (UTR) of ZMYND8. (H_–_J) Ectopically expressed GFP-ZMYND8 rescues defective CHD4 damage accrual in ZMYND8-depleted cells. U2OS cells stably expressing GFP-ZMYND8 were treated with si3′ UTR targeting endogenous but not GFP-tagged ZMYND8, which lacks the 3′ UTR. (I) Quantification of G and H performed as in F. n = 2. (J) Western blot analysis of samples from G and H with the indicated antibodies. Note: 3′ UTR siRNA targeting ZMYND8 depletes endogenous but not GFP-tagged ZMYND8.
Figure 5.
ZMYND8 identifies damage within transcriptionally active chromatin to promote HR. (A) Recruitment of ZMYND8 to damaged chromatin requires active transcription. Cells were analyzed as in Figure 2J with or without treatment with the transcriptional inhibitor DRB. (B) Quantification of A. Error bars indicate SEM; n > 10. (C) Recruitment of CHD4 to damaged chromatin requires active transcription. Cells were analyzed as in Figure 4E with or without DRB treatment. (D,E) ZMYND8 promotes RAD51 loading at HR-prone DSB sites within active chromatin. Samples from DlvA cells containing site-specific AsiSI-induced DSBs were analyzed by ChIP analysis for the HR factor RAD51 or NHEJ factor XRCC4 as described previously (Aymard et al. 2014). DSB I and DSB III represent HR-prone DSB sites, while DSB 1 and DSB 2 represent Non-HR-prone DSB sites. ChIP efficiency was measured by percentage of input, and mock versus antibody-containing data are graphed for each DSB from siControl and siZMYND8 #1 samples. (E) RAD51/XRCC4 ratios of data obtained in D.
Figure 6.
ZMYND8 and CHD4 mediate transcriptional repression upon DNA damage. (A) Scheme of nascent transcription analysis by 5-ethynyl uridine (5-EU) monitoring following laser damage. (B) ZMYND8 promotes transcriptional repression following laser damage. Cells treated with control or ZMYND8 siRNAs were subjected to the scheme shown in A and analyzed by immunofluorescence. γH2AX marks DNA damage. (C) Quantification of 5-EU and γH2AX fluorescence intensity from B. Measurements of fluorescent intensity along lines perpendicular to the laser damage, which contained both damaged and undamaged regions, were obtained. Values were normalized to undamaged regions. Error bars indicate SEM; n > 10. (D) CHD4 but not LSD1 is required for transcriptional repression following laser damage. Experiments performed as in B. (E) Quantification of D as in C. (F) Scheme of U2OS DSB reporter cells adapted by permission from Macmillan Publishers Ltd. from Tang et al. (2013), © 2013. Shield-1 and 4-OHT regulate the Fok1 nuclease, which induces DSBs upstream of reporter genes within LacO repeats. Doxycycline induces transcription of the reporter gene, allowing transcriptional repression upon DSB induction to be measured by quantitative PCR (qPCR). (G) ZMYND8 and CHD4, but not LSD1, regulate transcriptional repression at DSBs. The system from F was analyzed in cells treated with the indicated siRNAs. Error bars indicate SEM; n = 4. _P_-values were calculated using Student’s _t_-test. (H) ZMYND8 and CHD4 promote RAD51 loading at DSBs. RAD51 loading by immunofluorescence in siControl, siZMYND8, and siCHD4 cells was analyzed 3 h after DSB induction using a FokI-inducible DSB system (Tang et al. 2013). (I) Quantification of H. Error bars indicate SEM; n = 3. siRNA #1 was used for siZMYND8 treatments.
Figure 7.
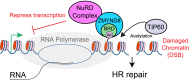
Model of DNA damage recognition pathway by ZMYND8. Upon DNA damage, ZMYND8 interacts with the NuRD complex. ZMYND8 recognizes TIP60-dependent acetylations (e.g., H4Ac) with its BRD, including within actively transcribing damaged chromatin, resulting in recruitment of the NuRD complex to these damage sites. The TIP60-dependent recruitment of the NuRD complex promotes transcriptional silencing that facilitates repair by HR, including within transcriptionally active damaged chromatin.
Similar articles
- Selective Recognition of H3.1K36 Dimethylation/H4K16 Acetylation Facilitates the Regulation of All-trans-retinoic Acid (ATRA)-responsive Genes by Putative Chromatin Reader ZMYND8.
Adhikary S, Sanyal S, Basu M, Sengupta I, Sen S, Srivastava DK, Roy S, Das C. Adhikary S, et al. J Biol Chem. 2016 Feb 5;291(6):2664-81. doi: 10.1074/jbc.M115.679985. Epub 2015 Dec 11. J Biol Chem. 2016. PMID: 26655721 Free PMC article. - Histone demethylase KDM5A regulates the ZMYND8-NuRD chromatin remodeler to promote DNA repair.
Gong F, Clouaire T, Aguirrebengoa M, Legube G, Miller KM. Gong F, et al. J Cell Biol. 2017 Jul 3;216(7):1959-1974. doi: 10.1083/jcb.201611135. Epub 2017 Jun 1. J Cell Biol. 2017. PMID: 28572115 Free PMC article. - ZMYND8 Co-localizes with NuRD on Target Genes and Regulates Poly(ADP-Ribose)-Dependent Recruitment of GATAD2A/NuRD to Sites of DNA Damage.
Spruijt CG, Luijsterburg MS, Menafra R, Lindeboom RG, Jansen PW, Edupuganti RR, Baltissen MP, Wiegant WW, Voelker-Albert MC, Matarese F, Mensinga A, Poser I, Vos HR, Stunnenberg HG, van Attikum H, Vermeulen M. Spruijt CG, et al. Cell Rep. 2016 Oct 11;17(3):783-798. doi: 10.1016/j.celrep.2016.09.037. Cell Rep. 2016. PMID: 27732854 - Bromodomain proteins: repairing DNA damage within chromatin.
Chiu LY, Gong F, Miller KM. Chiu LY, et al. Philos Trans R Soc Lond B Biol Sci. 2017 Oct 5;372(1731):20160286. doi: 10.1098/rstb.2016.0286. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28847823 Free PMC article. Review. - The nucleosome: orchestrating DNA damage signaling and repair within chromatin.
Agarwal P, Miller KM. Agarwal P, et al. Biochem Cell Biol. 2016 Oct;94(5):381-395. doi: 10.1139/bcb-2016-0017. Epub 2016 Apr 13. Biochem Cell Biol. 2016. PMID: 27240007 Review.
Cited by
- Cornelia de Lange syndrome-associated mutations cause a DNA damage signalling and repair defect.
Olley G, Pradeepa MM, Grimes GR, Piquet S, Polo SE, FitzPatrick DR, Bickmore WA, Boumendil C. Olley G, et al. Nat Commun. 2021 May 25;12(1):3127. doi: 10.1038/s41467-021-23500-6. Nat Commun. 2021. PMID: 34035299 Free PMC article. - Epigenetically Downregulated Breast Cancer Gene 2 through Acetyltransferase Lysine Acetyltransferase 2B Increases the Sensitivity of Colorectal Cancer to Olaparib.
Chen S, Allgayer H. Chen S, et al. Cancers (Basel). 2023 Nov 25;15(23):5580. doi: 10.3390/cancers15235580. Cancers (Basel). 2023. PMID: 38067284 Free PMC article. - Lysine Acetylation, Cancer Hallmarks and Emerging Onco-Therapeutic Opportunities.
Hu M, He F, Thompson EW, Ostrikov KK, Dai X. Hu M, et al. Cancers (Basel). 2022 Jan 11;14(2):346. doi: 10.3390/cancers14020346. Cancers (Basel). 2022. PMID: 35053509 Free PMC article. Review. - Lysine Acetylation Goes Global: From Epigenetics to Metabolism and Therapeutics.
Ali I, Conrad RJ, Verdin E, Ott M. Ali I, et al. Chem Rev. 2018 Feb 14;118(3):1216-1252. doi: 10.1021/acs.chemrev.7b00181. Epub 2018 Feb 6. Chem Rev. 2018. PMID: 29405707 Free PMC article. Review. - Senataxin resolves RNA:DNA hybrids forming at DNA double-strand breaks to prevent translocations.
Cohen S, Puget N, Lin YL, Clouaire T, Aguirrebengoa M, Rocher V, Pasero P, Canitrot Y, Legube G. Cohen S, et al. Nat Commun. 2018 Feb 7;9(1):533. doi: 10.1038/s41467-018-02894-w. Nat Commun. 2018. PMID: 29416069 Free PMC article.
References
- Adam S, Polo SE, Almouzni G. 2013. Transcription recovery after DNA damage requires chromatin priming by the H3.3 histone chaperone HIRA. Cell 155: 94–106. - PubMed
- Aguilera A, Garcia-Muse T. 2013. Causes of genome instability. Annu Rev Genet 47: 1–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials