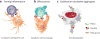TAM receptor signaling in immune homeostasis - PubMed (original) (raw)
Review
TAM receptor signaling in immune homeostasis
Carla V Rothlin et al. Annu Rev Immunol. 2015.
Abstract
The TAM receptor tyrosine kinases (RTKs)-TYRO3, AXL, and MERTK-together with their cognate agonists GAS6 and PROS1 play an essential role in the resolution of inflammation. Deficiencies in TAM signaling have been associated with chronic inflammatory and autoimmune diseases. Three processes regulated by TAM signaling may contribute, either independently or collectively, to immune homeostasis: the negative regulation of the innate immune response, the phagocytosis of apoptotic cells, and the restoration of vascular integrity. Recent studies have also revealed the function of TAMs in infectious diseases and cancer. Here, we review the important milestones in the discovery of these RTKs and their ligands and the studies that underscore the functional importance of this signaling pathway in physiological immune settings and disease.
Keywords: AXL; GAS6; MERTK; PROS1; TYRO3.
Figures
Figure 1
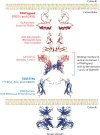
Domain organization of TAM receptors and ligands. The TAM ligands GAS6 and PROS1 (red) carry an N-terminal GLA domain that binds to PtdSer (green) exposed on the plasma membrane of cells in different settings, including apoptosis, immune activation, and coagulation. GLA domains are followed by four EGF-like repeats. At the C terminus, the TAM ligands carry two laminin G domains, of which domain 1 interacts with the Ig-like domains of the TAM RTKs to form a heterotetrameric complex. Ig-like domains are followed by two FNIII domains and an intracellular tyrosine kinase domain (blue). The domain structures show superimposed sequences of the three TAM receptors or two ligands and were predicted from either crystallographic data or sequence homology. The structure of the whole complex—including PtdSer, TAM ligands, and TAM receptors—remains unknown. (Abbreviations: EGF, epidermal growth factor; FNIII, fibronectin type III; RTK, receptor tyrosine kinase; TAM,
T
YRO3,
A
XL, and
M
ERTK.)
Figure 2
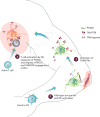
TAM signaling functions as a homeostatic negative feedback mechanism of the immune response. Patrolling dendritic cells (DCs) are activated upon pathogen encounter, leading to DC maturation ❶, migration to the draining lymph node, and induction of TAM RTK expression in DCs ❷. When DCs reach the draining lymph node, they present antigens to T cells, triggering T cell activation, exposure of PtdSer on the surface of activated T cells, and display of the TAM agonist PROS1. Once activated, T cell–derived PROS1 reports back to the DC through the inhibitory TAM RTKs in DCs ❸.
Figure 3
A triad of TAM functions in the homeostasis of the immune response. (a) TAM RTK signaling is engaged at the T cell–dendritic cell interface, whereby activated T cells expose PtdSer on their surface and express the TAM agonist PROS1 to activate the TAM RTKs in dendritic cells and regulate the magnitude of the immune response. (b) TAM RTK signaling mediates the phagocytosis of apoptotic cells, which is also known as efferocytosis. Apoptotic cells and membranes expose PtdSer on their surfaces. Binding of PtdSer by the TAM agonists triggers the activation of TAM RTKs in phagocytes such as macrophages. (c) TAM RTK signaling promotes the stabilization of platelet aggregates upon vascular injury. Activated platelets expose PtdSer on their surface that functions as a platform for the binding of various GLA-containing proteins, including the TAM agonists. Platelets also express the TAM RTKs, and activation of these receptors potentiates platelet aggregation and clot retraction. TAM RTK signaling also functions in endothelial and vascular smooth muscle cells (see text) to promote wound healing of the damaged vasculature.
Figure 4
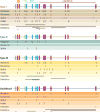
Schematic representation of PROS1 mutations, with the frequency of mutations in specific introns and exons of the PROS1 gene subdivided according to the type of PROS1 deficiency. Those mutations in PROS1 for which available clinical data are not sufficient for their classification into type I/II or III deficiency are presented as undefined. (Abbreviations: EGF, epidermal growth factor; TSR, thrombin-sensitive region.)
Figure 5
Contrasting functions of TAM signaling in viral infection. Enveloped viruses employ apoptotic mimicry, or the exposure of PtdSer on the viral envelope, to hijack the TAM pathway in dendritic cells and promote infection. Exposed PtdSer on the viral envelope potentiates the activation of TAM RTKs by the TAM agonists, leading to the suppression of the antiviral type I IFN response and favoring the infection of dendritic cells in vitro. Whether a similar mechanism occurs in vivo remains ill-defined (left). In contrast, TAM RTK signaling can favor the cross-presentation of viral antigens and lead to decreased viral infection in vivo. Phagocytosis of virally infected apoptotic cells in a TAM RTK–dependent manner leads to cross-presentation and the induction of a protective antiviral adaptive immune response (right).
Figure 6
Immunological functions of TAM signaling at the tumor-stroma interface. (a) TAM signaling can favor tumor growth in cancer through two independent mechanisms. Tumor-associated macrophages express the TAM agonist GAS6 and promote tumor growth through the activation of oncogenic TAM signaling in tumor cells (left). Activation of MERTK in tumor-associated macrophages leads to an immunosuppressive cytokine environment, decreased antitumor CD8+ T cell responses, and increased tumor growth. (b) In contrast, in colitis-associated cancer, TAM RTK signaling in intestinal lamina propria macrophages promotes an anti-inflammatory environment that limits chronic inflammation and associated tumors. The absence of this RTK pathway favors a pro-inflammatory environment in the colon and an increased incidence of colitis-associated cancer.
Figure 7
The TAMing of inflammation (with apologies to William Shakespeare). The illustration depicts the intimate interactions at the interface of the innate and adaptive immune responses, where antigen-presenting cells of the innate immune arm render the adaptive immune response. Upon activation, adaptive immune cells erase or limit the innate immune response.
Similar articles
- TAM receptors in phagocytosis: Beyond the mere internalization of particles.
Burstyn-Cohen T, Fresia R. Burstyn-Cohen T, et al. Immunol Rev. 2023 Oct;319(1):7-26. doi: 10.1111/imr.13267. Epub 2023 Aug 19. Immunol Rev. 2023. PMID: 37596991 Review. - Galectin-3 Stimulates Tyro3 Receptor Tyrosine Kinase and Erk Signalling, Cell Survival and Migration in Human Cancer Cells.
Al Kafri N, Hafizi S. Al Kafri N, et al. Biomolecules. 2020 Jul 11;10(7):1035. doi: 10.3390/biom10071035. Biomolecules. 2020. PMID: 32664510 Free PMC article. - Tyro3, Axl, and Mertk receptor signaling in inflammatory bowel disease and colitis-associated cancer.
Rothlin CV, Leighton JA, Ghosh S. Rothlin CV, et al. Inflamm Bowel Dis. 2014 Aug;20(8):1472-80. doi: 10.1097/MIB.0000000000000050. Inflamm Bowel Dis. 2014. PMID: 24846720 Free PMC article. Review. - The role of TAM family receptors and ligands in the nervous system: From development to pathobiology.
Shafit-Zagardo B, Gruber RC, DuBois JC. Shafit-Zagardo B, et al. Pharmacol Ther. 2018 Aug;188:97-117. doi: 10.1016/j.pharmthera.2018.03.002. Epub 2018 Mar 4. Pharmacol Ther. 2018. PMID: 29514053 Free PMC article. Review. - The TAM Subfamily of Receptor Tyrosine Kinases: The Early Years.
Prieto AL, Lai C. Prieto AL, et al. Int J Mol Sci. 2024 Mar 16;25(6):3369. doi: 10.3390/ijms25063369. Int J Mol Sci. 2024. PMID: 38542343 Free PMC article. Review.
Cited by
- TAM Receptor Inhibition-Implications for Cancer and the Immune System.
Aehnlich P, Powell RM, Peeters MJW, Rahbech A, Thor Straten P. Aehnlich P, et al. Cancers (Basel). 2021 Mar 10;13(6):1195. doi: 10.3390/cancers13061195. Cancers (Basel). 2021. PMID: 33801886 Free PMC article. Review. - Carcinogenesis: Failure of resolution of inflammation?
Fishbein A, Hammock BD, Serhan CN, Panigrahy D. Fishbein A, et al. Pharmacol Ther. 2021 Feb;218:107670. doi: 10.1016/j.pharmthera.2020.107670. Epub 2020 Sep 3. Pharmacol Ther. 2021. PMID: 32891711 Free PMC article. Review. - Glial Cell-Mediated Neuroinflammation in Alzheimer's Disease.
Al-Ghraiybah NF, Wang J, Alkhalifa AE, Roberts AB, Raj R, Yang E, Kaddoumi A. Al-Ghraiybah NF, et al. Int J Mol Sci. 2022 Sep 12;23(18):10572. doi: 10.3390/ijms231810572. Int J Mol Sci. 2022. PMID: 36142483 Free PMC article. Review. - TAM Receptor Pathways at the Crossroads of Neuroinflammation and Neurodegeneration.
Tondo G, Perani D, Comi C. Tondo G, et al. Dis Markers. 2019 Sep 15;2019:2387614. doi: 10.1155/2019/2387614. eCollection 2019. Dis Markers. 2019. PMID: 31636733 Free PMC article. Review. - Ginseng polysaccharides: Potential antitumor agents.
Tao R, Lu K, Zong G, Xia Y, Han H, Zhao Y, Wei Z, Lu Y. Tao R, et al. J Ginseng Res. 2023 Jan;47(1):9-22. doi: 10.1016/j.jgr.2022.07.002. Epub 2022 Jul 16. J Ginseng Res. 2023. PMID: 36644386 Free PMC article. Review.
References
- Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–75. - PubMed
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–82. - PubMed
- Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–96. - PubMed
- Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. - PubMed
- Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6:728–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous