The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer - PubMed (original) (raw)
doi: 10.1038/mt.2015.10. Epub 2015 Jan 19.
Shigehiro Yagishita 2, Keitaro Hagiwara 3, Yusuke Yoshioka 3, Nobuyoshi Kosaka 3, Fumitaka Takeshita 3, Tomohiro Fujiwara 3, Koji Tsuta 4, Hiroshi Nokihara 2, Tomohide Tamura 2, Hisao Asamura 5, Makoto Kawaishi 6, Kazuyoshi Kuwano 6, Takahiro Ochiya 3
Affiliations
- PMID: 25597412
- PMCID: PMC4395779
- DOI: 10.1038/mt.2015.10
The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer
Yu Fujita et al. Mol Ther. 2015 Apr.
Abstract
Programmed cell death ligand-1 (PD-L1) has recently gained considerable attention for its role in tumor immune escape. Here, we identify a miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer (NSCLC), independent of immunoinhibitory signals. miR-197 is downregulated in platinum-resistant NSCLC specimens, resulting in the promotion of chemoresistance, tumorigenicity, and pulmonary metastasis in vitro and in vivo. Mechanistic investigations reveal that a miR-197-mediated CKS1B/STAT3 axis exerts tumor progression regulated by various oncogenic genes (Bcl-2, c-Myc, and cyclin D1), and PD-L1 is a putative biomarker of this axis. Furthermore, we demonstrate that a miR-197 mimic sensitizes PD-L1(high) drug-resistant cells to chemotherapy. These results indicate that the biological interaction between PD-L1 and chemoresistance occurs through the microRNA regulatory cascade. More importantly, expression levels of miR-197 are inversely correlated with PD-L1 expression (n = 177; P = 0.026) and are associated with worse overall survival (P = 0.015). Our discoveries suggest that the miR-197/CKS1B/STAT3-mediated network can drive tumor PD-L1 expression as a biomarker of this cascade, and miR-197 replacement therapy may be a potential treatment strategy for chemoresistant NSCLC.
Figures
Figure 1
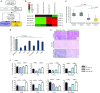
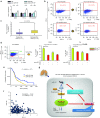
miR-197 is a novel miRNA related to chemoresistance and survival in NSCLC. (a) Schema for the patient selection of a cohort study for miRNA microarray. (b) A heat map of miRNA microarray analysis revealed differentially expressed miRNAs in tumor and adjacent normal tissues in the two groups. (c) qRT-PCR analysis of the expression levels of miR-197 in lung tumors and adjacent normal tissues. (d) qRT-PCR analysis of the expression levels of miR-197 in human lung cancer cell lines. (e) miR-197-specific probe and scramble control probe were hybridized in situ with lung adenocarcinoma tissue. Original magnification, ×100. (f) IC50 after transient transfection of each miRNA and CDDP and TXL treatment. *P < 0.05; **P < 0.01. CDDP, cisplatin; HE, hematoxylin and eosin; IC50, concentration of drug needed to inhibit cell growth by 50%; LNA, locked nucleic acid; miRNA, microRNA; NC, negative control; NSCLC, non-small-cell lung cancer; qRT-PCR, quantitative reverse transcription-PCR; TXL, paclitaxel.
Figure 2
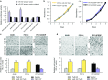
miR-197 regulates malignant phenotypes of lung cancer cells in vitro. (a) The luciferase activity of A549-miR-197-TuD, PC14-miR-197-TuD, and PC14CDDP-miR-197 cells was compared to that of each control cell line in response to a miR-197 sensor vector or the mutated vector. (b) No significant effect of decreased or increased miR-197 levels on the growth rate of PC14 or PC14CDDP. (c and d) Representative images (upper panels, pictures) and quantification (lower panels, graphs) of the effect on (c) cell invasion and (d) migration. The invasive or migration values were normalized to the values from control cells (scale bar = 100 μm). *P < 0.05; **P < 0.01. NC, negative control.
Figure 3
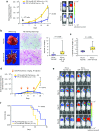
Knockdown of miR-197 promotes tumorigenicity, pulmonary metastasis, and chemoresistance in vivo. (a) The bioluminescent change emitted from the whole bodies of the mice bearing PC14-miR-197-TuD-luc or PC14-TuD-NC-luc cells (10 mice per group) (P = 0.005) (left graph) and representative bioluminescence images of lung tumor growth on days 7 and 28 (right panel). (b) Representative pictures of murine whole lung (left) and hematoxylin/eosin (HE) staining of the right lung are presented (right graph) (scale bar = 100 μm). Arrows indicate primary lung nodules, and triangles indicate the surface of metastatic nodules. The number of visible surface metastatic lesions in mice (10 mice per group) was significantly increased in the miR-197-attenuated group (P = 0.039). (c) The ratio of lung weights to total body weight in mice (10 mice per group) (P = 0.006). (d) The bioluminescent change emitted from the whole bodies of the mice bearing PC14-miR-197-TuD-luc or PC14-TuD-NC-luc cells (10 mice per group) after repeated intraperitoneal injections (IP injections) of CDDP (P = 0.016). (e) Representative bioluminescence images of lung tumor growth in each group after repeated administration of CDDP. (f) The overall survival rates in each group were estimated by the Kaplan–Meier method (P = 0.036). CDDP, cisplatin.
Figure 4
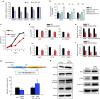
miR-197 targets the cyclin-dependent kinase CKS1B and regulates CKS1B/STAT3 signaling. (a) Dual-luciferase assays demonstrating that the repression of candidate genes by miR-197 was measured. (b) qRT-PCR analyses of mRNA levels of CKS1B in indicated cells treated with each miRNA. (c) Cell proliferation assay in A549 cells after transfection of each siRNA. (d) IC50 (CDDP or TXL) after transfection of each siRNA. (e and f) Quantification of the effect on (e) cell invasion and (f) migration after transfection of each siRNA. (g) The PD-L1 promoter regulatory module. A STAT3 binding site is indicated in bold. This region was cloned into a pGL-luciferase vector (pGL4-PD-L1p). Analyses of pGL4-PD-L1p luciferase activity in PC14CDDP cells treated with STAT3-siRNA, LNA-miR-197, or each NC. (h) Western blot analyses of target genes related to CKS1B/STAT3 signaling after treatment with LNA-miR-197 or control in PC14CDDP cells. *P < 0.05; **P < 0.01. CDDP, cisplatin; IC50, concentration of drug needed to inhibit cell growth by 50%; LNA, locked nucleic acid; miRNA, microRNA; mRNA, messenger RNA; NC, negative control; PD-L1, programmed cell death ligand-1; qRT-PCR, quantitative reverse transcription-PCR; siRNA, small interfering RNA; TXL, paclitaxel.
Figure 5
PD-L1 is a putative biomarker regulated by the miR-197/CKS1B/STAT3 signaling cascade in NSCLC. (a) qRT-PCR analyses of the mRNA levels of PD-L1 treated with each miRNA. (b) FCM analyses of PD-L1 expression induced by knockdown or overexpression of miR-197 in PC14 or PC14CDDP cells. (c) qRT-PCR analyses of mRNA levels of PD-L1 in lung tumor samples of the cohort study (P = 0.015). (d) FCM cell sorting to purify PD-L1high and PD-L1low cellular subsets from PC14CDDP to estimate the IC50 (CDDP or TXL) of PD-L1high and PD-L1low cells after transfection of each miRNA. (e) Survival outcomes of miR-197 expression in a validation cohort study (P = 0.015, n = 177). (f) Scatter plots between the expression of miR-197 and PD-L1 gene expression (P = 0.026, n = 177). (g) Scheme of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant NSCLC. The miR-197/CKS1B/STAT3 axis regulates cancer progression, and tumor PD-L1 expression as a putative biomarker of this cascade. *P < 0.05; **P < 0.01. CDDP, cisplatin; FCM, flow cytometry; IC50, concentration of drug needed to inhibit cell growth by 50%; miRNA, microRNA; mRNA, messenger RNA; NC, negative control; NSCLC, non-small-cell lung cancer; PD-L1, programmed cell death ligand-1; qRT-PCR, quantitative reverse transcription-PCR; TXL, paclitaxel.
Similar articles
- 3-O-(Z)-coumaroyloleanolic acid overcomes Cks1b-induced chemoresistance in lung cancer by inhibiting Hsp90 and MEK pathways.
Wang H, Sun M, Guo J, Ma L, Jiang H, Gu L, Wen H, Liao S, Chen J, Zeng B, Li Y, Li Y, Yu X, Feng Y, Zhou Y. Wang H, et al. Biochem Pharmacol. 2017 Jul 1;135:35-49. doi: 10.1016/j.bcp.2017.03.007. Epub 2017 Mar 11. Biochem Pharmacol. 2017. PMID: 28288818 - miR-124 modulates gefitinib resistance through SNAI2 and STAT3 in non-small cell lung cancer.
Hu FY, Cao XN, Xu QZ, Deng Y, Lai SY, Ma J, Hu JB. Hu FY, et al. J Huazhong Univ Sci Technolog Med Sci. 2016 Dec;36(6):839-845. doi: 10.1007/s11596-016-1672-x. Epub 2016 Dec 7. J Huazhong Univ Sci Technolog Med Sci. 2016. PMID: 27924500 - PD-L1 on peripheral blood T lymphocytes is prognostic in patients with non-small cell lung cancer (NSCLC) treated with EGFR inhibitors.
Meniawy TM, Lake RA, McDonnell AM, Millward MJ, Nowak AK. Meniawy TM, et al. Lung Cancer. 2016 Mar;93:9-16. doi: 10.1016/j.lungcan.2015.12.006. Epub 2015 Dec 30. Lung Cancer. 2016. PMID: 26898608 - Non-Small Cell Lung Cancer, PD-L1, and the Pathologist.
Kerr KM, Nicolson MC. Kerr KM, et al. Arch Pathol Lab Med. 2016 Mar;140(3):249-54. doi: 10.5858/arpa.2015-0303-SA. Arch Pathol Lab Med. 2016. PMID: 26927720 Review. - Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for Non-Small Cell Lung Cancer.
He J, Hu Y, Hu M, Li B. He J, et al. Sci Rep. 2015 Aug 17;5:13110. doi: 10.1038/srep13110. Sci Rep. 2015. PMID: 26279307 Free PMC article. Review.
Cited by
- The Roles of MicroRNA in Lung Cancer.
Wu KL, Tsai YM, Lien CT, Kuo PL, Hung AJ. Wu KL, et al. Int J Mol Sci. 2019 Mar 31;20(7):1611. doi: 10.3390/ijms20071611. Int J Mol Sci. 2019. PMID: 30935143 Free PMC article. Review. - Arsenic sulfide reverses cisplatin resistance in non-small cell lung cancer in vitro and in vivo through targeting PD-L1.
Tian W, Sun Y, Cheng Y, Ma X, Du W, Shi W, Guo Q. Tian W, et al. Thorac Cancer. 2021 Oct;12(19):2551-2563. doi: 10.1111/1759-7714.14136. Epub 2021 Sep 1. Thorac Cancer. 2021. PMID: 34469060 Free PMC article. - Integrated analysis of programmed cell death ligand 1 expression reveals increased levels in high-grade glioma.
Hölzl D, Hutarew G, Zellinger B, Schlicker HU, Schwartz C, Winkler PA, Sotlar K, Kraus TFJ. Hölzl D, et al. J Cancer Res Clin Oncol. 2021 Aug;147(8):2271-2280. doi: 10.1007/s00432-021-03656-w. Epub 2021 May 8. J Cancer Res Clin Oncol. 2021. PMID: 33963441 Free PMC article. - MLLT11/AF1q boosts oncogenic STAT3 activity through Src-PDGFR tyrosine kinase signaling.
Park J, Kim S, Joh J, Remick SC, Miller DM, Yan J, Kanaan Z, Chao JH, Krem MM, Basu SK, Hagiwara S, Kenner L, Moriggl R, Bunting KD, Tse W. Park J, et al. Oncotarget. 2016 Jul 12;7(28):43960-43973. doi: 10.18632/oncotarget.9759. Oncotarget. 2016. PMID: 27259262 Free PMC article. - The Importance of the Immune System and Molecular Cell Signaling Pathways in the Pathogenesis and Progression of Lung Cancer.
Smok-Kalwat J, Mertowska P, Mertowski S, Smolak K, Kozińska A, Koszałka F, Kwaśniewski W, Grywalska E, Góźdź S. Smok-Kalwat J, et al. Int J Mol Sci. 2023 Jan 12;24(2):1506. doi: 10.3390/ijms24021506. Int J Mol Sci. 2023. PMID: 36675020 Free PMC article. Review.
References
- Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: new biological insights and recent therapeutic advances. CA Cancer J Clin. 2011;61:91–112. - PubMed
- Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous