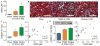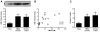Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease - PubMed (original) (raw)
Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease
W H Wilson Tang et al. Circ Res. 2015.
Abstract
Rationale: Trimethylamine-N-oxide (TMAO), a gut microbial-dependent metabolite of dietary choline, phosphatidylcholine (lecithin), and l-carnitine, is elevated in chronic kidney diseases (CKD) and associated with coronary artery disease pathogenesis.
Objective: To both investigate the clinical prognostic value of TMAO in subjects with versus without CKD, and test the hypothesis that TMAO plays a direct contributory role in the development and progression of renal dysfunction.
Methods and results: We first examined the relationship between fasting plasma TMAO and all-cause mortality over 5-year follow-up in 521 stable subjects with CKD (estimated glomerular filtration rate, <60 mL/min per 1.73 m(2)). Median TMAO level among CKD subjects was 7.9 μmol/L (interquartile range, 5.2-12.4 μmol/L), which was markedly higher (P<0.001) than in non-CKD subjects (n=3166). Within CKD subjects, higher (fourth versus first quartile) plasma TMAO level was associated with a 2.8-fold increased mortality risk. After adjustments for traditional risk factors, high-sensitivity C-reactive protein, estimated glomerular filtration rate, elevated TMAO levels remained predictive of 5-year mortality risk (hazard ratio, 1.93; 95% confidence interval, 1.13-3.29; P<0.05). TMAO provided significant incremental prognostic value (net reclassification index, 17.26%; P<0.001 and differences in area under receiver operator characteristic curve, 63.26% versus 65.95%; P=0.036). Among non-CKD subjects, elevated TMAO levels portend poorer prognosis within cohorts of high and low cystatin C. In animal models, elevated dietary choline or TMAO directly led to progressive renal tubulointerstitial fibrosis and dysfunction.
Conclusions: Plasma TMAO levels are both elevated in patients with CKD and portend poorer long-term survival. Chronic dietary exposures that increase TMAO directly contributes to progressive renal fibrosis and dysfunction in animal models.
Keywords: cardiovascular diseases; chronic kidney disease; fibrosis; gut microbiota; trimethylamine N-oxide.
© 2014 American Heart Association, Inc.
Figures
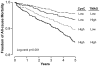
Figure 1. Prognostic Value of Plasma trimethylamine _N_-oxide (TMAO) Levels in the CKD Cohort
In a cohort of stable cardiac patients undergoing elective diagnostic coronary evaluation, subjects with underlying Stage 3+ chronic kidney disease demonstrated higher levels of fasting plasma TMAO than those with no CKD (p<0.01, Panel A). Increasing quartiles fasting plasma TMAO levels portend increased risk for all-cause mortality at 5 years in patients with CKD (n=521, Panel B).
Figure 2. Comparative Prognostic Value of Plasma TMAO and Cystatin C in the Non-CKD Cohort
Subjects with elevated cystatin C (>0.9 mg/dL) and TMAO (>3.4 μM) had the highest 5-year mortality risk in this non-CKD cohort (n=3,188).
Figure 3. Dietary choline/TMAO Exposure Contributes to Progressive Renal Fibrosis in Murine Model
Plasma TMAO (A) levels are increased after 6 week feeding TMAO (0.12%), or Choline (1.0%) diets vs chow (0.08% choline) fed mice. Representative Mason trichrome histology (B) quantitative morphometry (C) and its relationship with TMAO levels (D), SMAD3 activation by phosphorylation at serine 423/425 (E) and its relationship with TMAO levels (F) in mouse kidneys after 6 week feeding of chow (0.08% choline), TMAO (0.12%), and Choline (1.0%) diets. Scale bar represents 100 um. **P<0.01 vs. chow fed, n≥5 mice per group.
Figure 4. Dietary choline/TMAO Exposure Contributes to Progressive Renal Injury and Dysfunction in Murine Model
Immunoblot of KIM-1 expression (A) and its relationship with TMAO levels (B) in mouse kidneys after 6 week feeding of chow (0.08% choline), TMAO (0.12%), and Choline (1.0%) diets. Also shown are plasma cystatin C levels (C) after 16 week feeding of chow, TMAO (0.12%), and Choline (1.0%) diets. **P<0.01 vs. chow fed, n≥5 mice per group.
Comment in
- TMAO is both a biomarker and a renal toxin.
Fogelman AM. Fogelman AM. Circ Res. 2015 Jan 30;116(3):396-7. doi: 10.1161/CIRCRESAHA.114.305680. Circ Res. 2015. PMID: 25634968 Free PMC article. No abstract available.
References
- Mafra D, Lobo JC, Barros AF, Koppe L, Vaziri ND, Fouque D. Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol. 2014;9:399–410. - PubMed
- Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. - PubMed
- Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: Sources, consequences, and therapy. Seminars in dialysis. 2002;15:329–337. - PubMed
- Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–1016. - PubMed
- Lee CT, Hsu CY, Tain YL, Ng HY, Cheng BC, Yang CC, Wu CH, Chiou TT, Lee YT, Liao SC. Effects of ast-120 on blood concentrations of protein-bound uremic toxins and biomarkers of cardiovascular risk in chronic dialysis patients. Blood Purif. 2014;37:76–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL103866/HL/NHLBI NIH HHS/United States
- R01 HL103931/HL/NHLBI NIH HHS/United States
- R01 DK106000/DK/NIDDK NIH HHS/United States
- R01 HL103866/HL/NHLBI NIH HHS/United States
- P01 HL076491/HL/NHLBI NIH HHS/United States
- P20HL113452/HL/NHLBI NIH HHS/United States
- P20 HL113452/HL/NHLBI NIH HHS/United States
- P01HL098055/HL/NHLBI NIH HHS/United States
- R01HL103931/HL/NHLBI NIH HHS/United States
- P01 HL098055/HL/NHLBI NIH HHS/United States
- P01HL076491/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials