The role of invasive trophoblast in implantation and placentation of primates - PubMed (original) (raw)
Review
The role of invasive trophoblast in implantation and placentation of primates
Anthony M Carter et al. Philos Trans R Soc Lond B Biol Sci. 2015.
Abstract
We here review the evolution of invasive placentation in primates towards the deep penetration of the endometrium and its arteries in hominoids. The strepsirrhine primates (lemurs and lorises) have non-invasive, epitheliochorial placentation, although this is thought to be derived from a more invasive type. In haplorhine primates, there is differentiation of trophoblast at the blastocyst stage into syncytial and cellular trophoblast. Implantation involves syncytiotrophoblast that first removes the uterine epithelium then consolidates at the basal lamina before continuing into the stroma. In later stages of pregnancy, especially in Old World monkeys and apes, cytotrophoblast plays a greater role in the invasive process. Columns of trophoblast cells advance to the base of the implantation site where they spread out to form a cytotrophoblastic shell. In addition, cytotrophoblasts advance into the lumen of the spiral arteries. They are responsible for remodelling these vessels to form wide, low-resistance conduits. In human and great apes, there is additional invasion of the endometrium and its vessels by trophoblasts originating from the base of the anchoring villi. Deep trophoblast invasion that extends remodelling of the spiral arteries to segments in the inner myometrium evolved in the common ancestor of gorilla, chimp and human.
Keywords: arterial remodelling; catarrhine primates; cytrotrophoblast; platyrrhine primates; strepsirhine primates; syncytiotrophoblast.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures
Figure 1.
Invasive trophoblast in platyrrhine primates. (a) Overview of the implantation site in the common marmoset (Callithrix jacchus) on day 13 of pregnancy. The amniotic cavity is well formed. At the extreme right is the edge of the implantation site, where mural trophoblast (mtr) overlies uterine epithelium. In the rest of the implantation site, the syncytial trophoblast has largely eliminated the uterine epithelium. Note the dilation of the superficial maternal vessels (mv). Scale bar, 74 μm. (b) Syncytial trophoblast from the central region of the implantation site of C. jacchus. Numerous lamellipodia abut the residual basal lamina (bl) of the uterine luminal epithelium. Scale bar, 2.4 μm. (c) Breach of the basal lamina in C. jacchus. A process from the syncytial trophoblast (long arrow) has penetrated the residual basal lamina (bl) of the uterine luminal epithelium. Note that the projection has reached but not penetrated the basal lamina underlying the endothelium of the maternal vessel. Scale bar, 2.2 μm. Adapted with permission from Enders & Lopata [20] Copyright © 1999 Wiley-Liss, Inc. (d) Transition between a uterine artery and an intraplacental capillary in C. jacchus at 60 days of pregnancy. The thick basal lamina (bl) beneath the capillary endothelium is clearly visible. No basal lamina is seen beneath the artery, which appears devoid of endothelium and is lined by a thick layer of cells that we suggest is trophoblast. Boyd Collection, Centre for Trophoblast Research. Cambridge. Scale bar, 105 μm. (e) Transition between a uterine artery and an intraplacental capillary in the white-fronted capuchin (Cebus gracilis). The arterial lumen appears to be lined by fibrin and beneath it is a thick pad of cells. Hill Collection, Museum für Naturkunde, Berlin. Scale bar, 100 μm. (Online version in colour.)
Figure 2.
Invasive trophoblast at the implantation site in Old World monkeys. (a) Implantation site of a baboon (Papio sp.) at 10 days of pregnancy. Dark cytotrophoblast cells differentiating to syncytial trophoblast are seen towards the endometrium and pale cytotrophoblast cells are adjacent to the blastocyst cavity. The trophoblast has removed the uterine epithelium. ICM: inner cell mass. Scale bar, 50 μm. (b) Detail of the same implantation site showing syncytial trophoblast entering a superficial maternal capillary. Scale bar, 6.4 μm. (c) Implantation site of a rhesus macaque (Macaca mulatta) at 15 days of pregnancy. Columns of cytotrophoblast cells (cc) are advancing into the uterine stroma, bypassing the epithelial plaques (pl). Scale bar, 56 μm. (d) By 24 days of pregnancy in the macaque, the cells have spread out to form a cytotrophoblastic shell. Scale bar, 80 μm. (Online version in colour.)
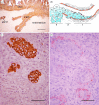
Figure 3.
Invasive trophoblast in the placental bed of Old World primates. (a) In the rhesus macaque (M. mulatta), the cytotrophoblastic shell is sharply delineated from the underlying decidua as demonstrated in this section from 50 days of gestation by immunostaining for cytokeratin (brown). A maternal artery completely remodelled by trophoblast occupies the centre of the field. The epithelium of a uterine gland is also immunopositive for cytokeratin, but there is no interstitial trophoblast. Scale bar, 1120 μm. (b) Diagram of a cytotrophoblast cell mass progressing into a maternal artery, against the flow of blood. The trophoblast shell would be located beyond the left side of the diagram. (1) Area of cytotrophoblast cell migration along the luminal surface of endothelium. The cytotrophoblast cells are adherent to the endothelium and to one another. (2) Zone of peripheral cytotrophoblast penetration through the tunica intima and into the tunica media. (3) Region of intramural cytotrophoblast hypertrophy and extracellular matrix synthesis. Modified from Enders [31]. (c) Invasion of spiral arteries by the endovascular route in M. mulatta at 15 days of gestation. Cytokeratin-positive trophoblast can be seen within the three upper coils of a spiral artery. Scale bar, 50 μm. (d) The smooth muscle is beginning to disperse in the wall of the three invaded segments, as shown by immunostaining of an adjacent section for desmin, but is intact in the two segments at the bottom of the field. Scale bar, 50 μm. (Online version in colour.)
Figure 4.
Invasive trophoblast in the placental bed of apes. (a) In gibbons, there is a continuous cytotrophoblastic cell as shown by immunostaining for cytokeratin 7/17 (black) in a Javan gibbon (Hylobates moloch). Hill Collection Museum für Naturkunde, Berlin. Scale bar, 200 μm. (b) Decidual–myometrial junction in the placental bed of a western gorilla (G. gorilla) in early pregnancy. Double immunostaining for smooth muscle α-actin (black) followed by cytokeratin (red) shows residual vascular smooth muscle and the presence of intramural trophoblast (circled area). Scale bar, 100 μm. Adapted from Pijnenborg et al. [56] with permission from Elsevier. (c) Cluster of spiral artery cross-sections at the decidual–myometrial junction of a chimpanzee (Pan troglodytes) early in pregnancy. Immunostaining for cytokeratin (black) shows the presence of interstitial trophoblast, but absence of endovascular trophoblast. The detachment of the endothelial layer from the vessel wall probably is artefactual. Scale bar, 100 μm. Adapted from Pijnenborg et al. [57] with permission from Elsevier. (d) Deep radial artery in a chimpanzee later in pregnancy. Immunostaining for cytokeratin (black) shows endovascular trophoblast invasion, but absence of clear intramural incorporation. Interstitial trophoblast is absent at this myometrial depth. Scale bar, 200 μm. Adapted from Pijnenborg et al. [57] with permission from Elsevier. (Online version in colour.)
Similar articles
- Transition from lacunar to villous stage of implantation in the macaque, including establishment of the trophoblastic shell.
Enders AC. Enders AC. Acta Anat (Basel). 1995;152(3):151-69. doi: 10.1159/000147694. Acta Anat (Basel). 1995. PMID: 7572026 - Evolution of invasive placentation with special reference to non-human primates.
Carter AM, Pijnenborg R. Carter AM, et al. Best Pract Res Clin Obstet Gynaecol. 2011 Jun;25(3):249-57. doi: 10.1016/j.bpobgyn.2010.10.010. Epub 2010 Nov 4. Best Pract Res Clin Obstet Gynaecol. 2011. PMID: 21056010 Review. - Deep placentation.
Pijnenborg R, Vercruysse L, Brosens I. Pijnenborg R, et al. Best Pract Res Clin Obstet Gynaecol. 2011 Jun;25(3):273-85. doi: 10.1016/j.bpobgyn.2010.10.009. Epub 2011 Jan 5. Best Pract Res Clin Obstet Gynaecol. 2011. PMID: 21212025 Review. - Epithelial-mesenchymal transition during trophoblast differentiation.
Vićovac L, Aplin JD. Vićovac L, et al. Acta Anat (Basel). 1996;156(3):202-16. doi: 10.1159/000147847. Acta Anat (Basel). 1996. PMID: 9124037 Review.
Cited by
- The Role of Interferon (IFN)-γ in Extravillous Trophoblast Cell (EVT) Invasion and Preeclampsia Progression.
Nurzadeh M, Ghalandarpoor-Attar SM, Ghalandarpoor-Attar SN, Rabiei M. Nurzadeh M, et al. Reprod Sci. 2023 May;30(5):1462-1469. doi: 10.1007/s43032-022-01110-x. Epub 2022 Oct 26. Reprod Sci. 2023. PMID: 36289172 Review. - Evolutionary divergence of embryo implantation in primates.
Siriwardena D, Boroviak TE. Siriwardena D, et al. Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210256. doi: 10.1098/rstb.2021.0256. Epub 2022 Oct 17. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36252209 Free PMC article. Review. - A hexa-species transcriptome atlas of mammalian embryogenesis delineates metabolic regulation across three different implantation modes.
Malkowska A, Penfold C, Bergmann S, Boroviak TE. Malkowska A, et al. Nat Commun. 2022 Jun 16;13(1):3407. doi: 10.1038/s41467-022-30194-x. Nat Commun. 2022. PMID: 35710749 Free PMC article. - CCN1 (CYR61) and CCN3 (NOV) signaling drives human trophoblast cells into senescence and stimulates migration properties.
Kipkeew F, Kirsch M, Klein D, Wuelling M, Winterhager E, Gellhaus A. Kipkeew F, et al. Cell Adh Migr. 2016 Mar 3;10(1-2):163-78. doi: 10.1080/19336918.2016.1139265. Epub 2016 Jan 8. Cell Adh Migr. 2016. PMID: 26744771 Free PMC article. - Reliability of Rodent and Rabbit Models in Preeclampsia Research.
Sakowicz A, Bralewska M, Kamola P, Pietrucha T. Sakowicz A, et al. Int J Mol Sci. 2022 Nov 18;23(22):14344. doi: 10.3390/ijms232214344. Int J Mol Sci. 2022. PMID: 36430816 Free PMC article. Review.
References
- Enders AC, Schlafke S. 1986. Implantation in nonhuman primates and in the human. In Comparative primate biology, vol. 3: reproduction and development (eds Dukelow WR, Erwin J.), pp. 291–310. New York, NY: Alan R. Liss Inc.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources