Proteomics: in pursuit of effective traumatic brain injury therapeutics - PubMed (original) (raw)
Review
Proteomics: in pursuit of effective traumatic brain injury therapeutics
Pavel N Lizhnyak et al. Expert Rev Proteomics. 2015 Feb.
Abstract
Effective traumatic brain injury (TBI) therapeutics remains stubbornly elusive. Efforts in the field have been challenged by the heterogeneity of clinical TBI, with greater complexity among underlying molecular phenotypes than initially conceived. Future research must confront the multitude of factors comprising this heterogeneity, representing a big data challenge befitting the coming informatics age. Proteomics is poised to serve a central role in prescriptive therapeutic development because it offers an efficient endpoint within which to assess post-TBI biochemistry. We examine rationale for multifactor TBI proteomic studies and the particular importance of temporal profiling in defining biochemical sequences and guiding therapeutic development. Finally, we offer perspective on repurposing biofluid proteomics to develop theragnostic assays with which to prescribe, monitor and assess pharmaceutics for improved translation and outcome for patients with TBI.
Keywords: TBI; brain injury; informatics; inhibitory synapses; post-translational modification; proteomics; temporal; theragnostics; therapeutics.
Figures
Figure 1. TBI pathobiology comprises a series of biochemical and physiological events that initiate and evolve in an individualized fashion
The response to TBI is dynamic and individualize based on a host of population- and injury-associated variables; yet, too often proteomic studies invoke static endpoint analysis. We must strive toward temporal analysis of the TBI proteome to effectively resolve the underlying processes of TBI and the extent to which they vary with key study factors.
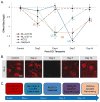
Figure 2. Decoding the temporal proteomic response to TBI: uncovered initiation cascade in remodeling the inhibitory synaptic network
Future applications of proteomics to TBI must consider the temporal dimensionality of the molecular response to TBI. For example, A. the initiation of inhibitory synaptic remodeling involves a series of post-translational events: (1) transient NL-2 ubiquitin signaling on K749 by day two; (2) transient NL-2 dephosphorylation on S714 by day four; (3) dissociation of KCC2 symporters from the membrane between days four and 14; (4) depolymerization of membrane-tethered gephyrin by day seven through 14. Mean±SE, n=6 per time point. B. NL-2 immunofluorescence depicting loss of somatic NL-2 staining (centered at dashed circles) at day two coinciding with K749 ubiquitin signaling, followed by synaptic NL-2 loss (red puncta) coinciding with S714 dephosphorylation at day four. NL-2 staining then recovers between seven and 14 days after TBI in time with recovery of S714 phosphorylation. Representative images of layer 4 somatosensory cortex; bar = 10 μm. C. Trajectory of molecular events in initiating inhibitory synaptic remodeling after TBI. CCI: controlled cortical impact; KCC2: potassium-chloride co-transporter 2; NL-2: neuroligin-2.
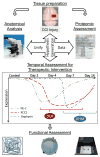
Figure 3. TBI proteomics guides novel intervention opportunities in a systems biology approach
Proteomics provides us with an efficient means to investigate the diverse biochemistry underlying TBI pathobiology. As a broad-scope endpoint measure, proteomics is conducive to discovery analysis when integrated with targeted anatomical, physiological and functional assessments as affirmatory evidence. Proteomics can inform, for example, on the molecular sequence underlying critical period inhibitory network remodeling within the first few weeks following injury. Pharmaceutics may then be selected based on the proteomic results. For example, DOI and DHM may be efficacious in maintaining the inhibitory synaptic network by mitigating TBI-induced declines in KCC2 symporters and polymerized gephyrin, respectively, when administered appropriately within the defined critical window.
References
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the united states: Emergency department visits, hospitalizations and deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010.
- Tsai YT, Wang CC, Leung PO, et al. Extracellular signal-regulated kinase 1/2 is involved in a tamoxifen neuroprotective effect in a lateral fluid percussion injury rat model. J Surg Res. 2014;189(1):106–116. - PubMed
- Zhou XM, Zhou ML, Zhang XS, et al. Resveratrol prevents neuronal apoptosis in an early brain injury model. J Surg Res. 2014;189(1):159–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources