Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: an in vitro and in vivo study - PubMed (original) (raw)
Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: an in vitro and in vivo study
H-Y Li et al. Cell Death Dis. 2015.
Abstract
Osteosarcoma is the most common primary malignant tumor of bone, the long-term survival of which has stagnated in the past several decades. Celastrol, a triterpene from traditional Chinese medicine, has been proved to possess potent anti-tumor effect on various cancers. However, the effect of celastrol on human osteosarcoma and the underlying mechanisms remains to be elucidated. We reported here that celastrol could inhibit cell proliferation by causing G2/M phase arrest. Exposure to celastrol resulted in the activation of caspase-3, -8, and -9, indicating that celastrol induced apoptosis through both extrinsic and intrinsic pathways. Autophagy occurred in celastrol-treated cells as evidenced by formation of autophagosome and accumulation of LC3B-II. The celastrol-induced cell death was remarkably restored by the combination of autophagy and apoptosis inhibitors. Furthermore, inhibition of apoptosis enhanced autophagy while suppression of autophagy diminished apoptosis. Celastrol also induced JNK activation and ROS generation. The JNK inhibitor significantly attenuated celastrol-triggered apoptosis and autophagy while ROS scavenger could completely reverse them. The ROS scavenger also prevented G2/M phase arrest and phosphorylation of JNK. Importantly, we found that celastrol had the similar effects on primary osteosarcoma cells. Finally, in vivo, celastrol suppressed tumor growth in the mouse xenograft model. Taken together, our results revealed that celastrol caused G2/M phase arrest, induced apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells. Celastrol is therefore a promising candidate for development of antitumor drugs targeting osteosarcoma.
Figures
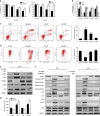
Figure 1
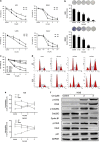
Celastrol inhibits cell proliferation and induces G2/M phase arrest in human osteosarcoma cells. (a) The anti-proliferative effect of celastrol on four osteosarcoma cell lines was determined by MTS. Cells were treated with various concentrations of celastrol for 24 and 48 h. Control group contained 0.1% DMSO. Data represented the mean of five replicates. Each performed in triplicate. (b) Colony formation assay of HOS and MG-63 cells with control or celastrol. (c) Comparison of the effect of celastrol on three normal human primary skin fibroblast samples with that on osteosarcoma cells for 24 h. (d, e) Celastrol induced G2/M phase arrest. Cells were treated with control or celastrol for 24 h and analyzed by flow cytometry. The percentage of cell cycle distribution was presented as the mean±S.D. from three independent experiments. (f) HOS cells were treated with celastrol for 24 h. The expressions of cell cycle-regulated proteins were measured by western blot. *P<0.05, significantly different compared with control
Figure 2
Evidence that celastrol induces apoptosis in osteosarcoma cells. (a) Apoptotic morphological changes were evaluated by fluorescent microscopy using Hoechst 33258 staining. Arrows indicate chromatin condensation and DNA fragmentation. Bar: 50 _μ_m. (b) HOS and MG-63 cells treated with celastrol were stained with annexin V-PE/7-AAD and analyzed by flow cytometry. The chart illustrates apoptosis proportion from three separate experiments. (c) The mitochondrial membrane potential was measured with JC-1 fluorescent probe and assessed by flow cytometry. The chart illustrates changes of JC-1 red/green rate from three independent experiments. (d, e) Cells were treated with various concentrations of celastrol for 24 h or incubated with celastrol (3 _μ_M) for different hours. The expressions of cleaved PARP, caspase-3, -8, -9, DR5 and Bid were determined by western blot. (f) Caspase activity assay of cells treated with various concentrations of celastrol for 24 h. (g) HOS cells were incubated with or without celastrol for 24 h after 2 h pre-treatment with caspase inhibitors, z-IETD-fmk (10 _μ_M), z-LEHD-fmk (40 _μ_M) or z-VAD-fmk (20 _μ_M). Then cells were stained with annexin V-PE/7-AAD and analyzed by flow cytometry. Results are expressed as the mean±S.D. from three independent experiments. *P<0.05 versus control, #P<0.05 versus celastrol treatment
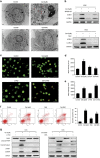
Figure 3
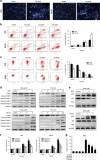
Celastrol induces autophagy, which contributes to cell death. (a) Cells were pretreated with z-VAD-fmk (20 _μ_M) for 2 h and then incubated with control or celastrol for 24 h. Cell viability was assessed by MTS. (b) The levels of AIF and Endo G in the mitochondria and cytosol were determined by western blot in HOS cells. (c) Cells were treated with various concentrations of celastrol for 24 h or incubated with celastrol (3 _μ_M) for different hours. The level of LC3B was measured by western blot. (d) Cells treated with or without celastrol for 24 h were collected and stained with acridine orange. Representative images of acridine orange-stained cells captured by fluorescent microscopy ( × 400) were shown. Bar: 50 _μ_m. (e) Transmission electron microscopy was utilized to observe the formation of autophagosome and ultrastructural change of nucleus. Arrows indicate autophagosomes containing intact and degraded cellular debris. Asterisks indicate nuclear condensation. Bar: 1 _μ_m. (f) z-VAD-fmk (20 _μ_M), 3-MA (2.5 mM for HOS, 5 mM for MG-63) or combination of them was added to cells 2 h before celastrol treatment. After 24 h, cell viability was determined. *P<0.05 versus control, #P<0.05 versus celastrol treatment
Figure 4
ROS generation and JNK activation are triggered by celastrol. (a, b) Cells were treated with celastrol for 12 h and then loaded with DCFH-DA for 30 min. The level of ROS was determined by fluorescence microscopy ( × 200) and flow cytometry. Representative images were presented. Quantitative analysis of ROS generation was shown in histograms. Bar: 200 _μ_m. *P<0.05 versus control, #P<0.05 versus celastrol treatment. (c) Cells were treated with various concentrations of celastrol for 24 h or incubated with celastrol (3 _μ_M) for different hours. Levels of phospho-JNK and total JNK were determined by western blot
Figure 5
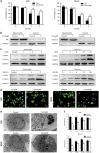
Roles of ROS and JNK in G2/M phase arrest and apoptosis induced by celastrol. HOS cells were preincubated with SP600125 (40 _μ_M) or NAC (5 mM) for 1 h, and then treated with celastrol (3 _μ_M) for 24 h. (a) Cell viability was measured by MTS. (b) Cell cycle was evaluated by flow cytometry. The percentage of cell cycle distribution was presented in histograms. (c, d) Induction of apoptosis and changes of mitochondrial membrane potential were assessed by flow cytometry. Quantitative analysis in histograms was presented. (e) The expressions of cell cycle-regulated proteins were measured by western blot. (f) Changes of apoptosis-related proteins. phospho-JNK and total JNK were measured by western blot. (g) The level of ROS was determined by flow cytometry. Quantitative analysis of ROS generation was shown in histograms. *P<0.05 versus control, #P<0.05 versus celastrol treatment
Figure 6
Roles of ROS and JNK in autophagy and the interplay between apoptosis and autophagy. HOS cells were preincubated with SP600125 (40 _μ_M), NAC (5 mM) for 1 h, or 3MA (2.5 mM), z-VAD-fmk (20 _μ_M) for 2 h, and then treated with celastrol (3 _μ_M) for 24 h. (a) Transmission electron microscopy was utilized to evaluate the changes of autophagosome and nucleus. Arrows indicate autophagosomes and asterisks indicate nuclear condensation. Bar: 1 _μ_m. (b) The expression of LC3B was analyzed by western blot. (c–e) The level of acridine orange staining was determined by fluorescence microscopy ( × 400) and flow cytometry. Representative images were presented. Quantitative analysis of red fluorescence representing acidic vesicles was shown in histograms. Bar: 50 _μ_m. (f) Cells were stained with annexin V-PE/7-AAD and analyzed by flow cytometry. The chart illustrates apoptosis proportion from three separate experiments. (g) Levels of LC3B, cleaved PARP and caspase-3 were assessed by western blot. *P<0.05 versus control, #P<0.05 versus celastrol treatment
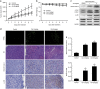
Figure 7
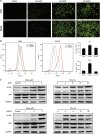
Celastrol inhibits growth of human osteosarcoma xenograft in vivo. HOS cells were inoculated subcutaneously in the right flank of BALB/c-nu mice. Intraperitoneal administration of vehicle or celastrol (1 or 2 mg/kg) daily was started when tumor volume reached around 200 mm3. When the tumors of control group reached around 1600 mm3, all mice were killed. (a, b) Tumor sizes and body weights were measured daily. (c) The levels of cleaved caspase-3, phospho-JNK and total JNK in tumor xenograft tissues were measured by western blot. (d) The apoptotic status of tumor tissues was assessed by TUNEL assay. H&E staining was used to evaluate the histology. The expression levels of cleaved caspase-3 and phospho-JNK were also examined by immunohistochemistry. Representative images were presented. Bar: 50 _μ_m. (e) Mean optical density of cleaved caspase-3 and phospho-JNK was quantified by Image Pro-Plus. *P<0.05, significantly different compared with control
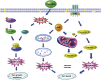
Figure 8
A schematic diagram of the pathways in which celastrol induces cell growth inhibition and cell death in osteosarcoma
Similar articles
- Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion.
Sun ZL, Dong JL, Wu J. Sun ZL, et al. Biomed Pharmacother. 2017 Jan;85:303-312. doi: 10.1016/j.biopha.2016.11.030. Epub 2016 Nov 26. Biomed Pharmacother. 2017. PMID: 27899257 - Honokiol induces apoptosis and autophagy via the ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro and in vivo.
Huang K, Chen Y, Zhang R, Wu Y, Ma Y, Fang X, Shen S. Huang K, et al. Cell Death Dis. 2018 Feb 6;9(2):157. doi: 10.1038/s41419-017-0166-5. Cell Death Dis. 2018. PMID: 29410403 Free PMC article. - Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo.
Wang H, Zhang T, Sun W, Wang Z, Zuo D, Zhou Z, Li S, Xu J, Yin F, Hua Y, Cai Z. Wang H, et al. Cell Death Dis. 2016 Jun 2;7(6):e2247. doi: 10.1038/cddis.2016.138. Cell Death Dis. 2016. PMID: 27253411 Free PMC article. - Celastrol as an emerging anticancer agent: Current status, challenges and therapeutic strategies.
Wang C, Dai S, Zhao X, Zhang Y, Gong L, Fu K, Ma C, Peng C, Li Y. Wang C, et al. Biomed Pharmacother. 2023 Jul;163:114882. doi: 10.1016/j.biopha.2023.114882. Epub 2023 May 15. Biomed Pharmacother. 2023. PMID: 37196541 Review. - Design and Synthesis of Novel Celastrol Derivatives as Potential Anticancer Agents against Gastric Cancer Cells.
Lei ZC, Li N, Yu NR, Ju W, Sun XN, Zhang XL, Dong HJ, Sun JB, Chen L. Lei ZC, et al. J Nat Prod. 2022 May 27;85(5):1282-1293. doi: 10.1021/acs.jnatprod.1c01236. Epub 2022 May 10. J Nat Prod. 2022. PMID: 35536757 Review.
Cited by
- Dihydrocelastrol induces antitumor activity and enhances the sensitivity of bortezomib in resistant multiple myeloma by inhibiting STAT3-dependent PSMB5 regulation.
Jin S, Li B, Zhang B, Gao X, Jia X, Xu L, Chang S, Hu K, Wang G, Xu Z, Zhang T, Song D, Yang G, Wu X, Zhu H, Huang C, Lu Y, Shi J, Zhu W, Chen G. Jin S, et al. Acta Biochim Biophys Sin (Shanghai). 2023 Dec 25;55(12):1884-1891. doi: 10.3724/abbs.2023260. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 38009004 Free PMC article. - Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells.
Chen X, Zhao Y, Luo W, Chen S, Lin F, Zhang X, Fan S, Shen X, Wang Y, Liang G. Chen X, et al. Theranostics. 2020 Aug 15;10(22):10290-10308. doi: 10.7150/thno.46728. eCollection 2020. Theranostics. 2020. PMID: 32929349 Free PMC article. - Metabolomics profiles delineate uridine deficiency contributes to mitochondria-mediated apoptosis induced by celastrol in human acute promyelocytic leukemia cells.
Zhang X, Yang J, Chen M, Li L, Huan F, Li A, Liu Y, Xia Y, Duan JA, Ma S. Zhang X, et al. Oncotarget. 2016 Jul 19;7(29):46557-46572. doi: 10.18632/oncotarget.10286. Oncotarget. 2016. PMID: 27374097 Free PMC article. - Celastrol Efficacy by Oral Administration in the Adjuvant-Induced Arthritis Model.
Cascão R, Vidal B, Carvalho T, Lopes IP, Romão VC, Goncalves J, Moita LF, Fonseca JE. Cascão R, et al. Front Med (Lausanne). 2020 Sep 8;7:455. doi: 10.3389/fmed.2020.00455. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33015082 Free PMC article. - Natural products for enhancing the sensitivity or decreasing the adverse effects of anticancer drugs through regulating the redox balance.
Sun Y, Li Q, Huang Y, Yang Z, Li G, Sun X, Gu X, Qiao Y, Wu Q, Xie T, Sui X. Sun Y, et al. Chin Med. 2024 Aug 20;19(1):110. doi: 10.1186/s13020-024-00982-2. Chin Med. 2024. PMID: 39164783 Free PMC article. Review.
References
- Raymond AK, Jaffe N. Osteosarcoma multidisciplinary approach to the management from the pathologist's perspective. Cancer Treat Res 2009; 152: 63–84. - PubMed
- Tao XL, Cush JJ, Garret M, Lipsky PE. A phase I study of ethyl acetate extract of the Chinese antirheumatic herb Tripterygium wilfordii Hook F in rheumatoid arthritis. J Rheumatol 2001; 28: 2160–2167. - PubMed
- Allison AC, Cacabelos R, Lombardi VRM, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease. Prog Neuro-Psychoph 2001; 25: 1341–1357. - PubMed
- Li H, Zhang YY, Huang XY, Sun YN, Jia YF, Li D. Beneficial effect of tripterine on systemic lupus erythematosus induced by active chromatin in BALB/c mice. Eur J Pharmacol 2005; 512: 231–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous