Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences - PubMed (original) (raw)
Review
Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences
Linda A Gallo et al. Diab Vasc Dis Res. 2015 Mar.
Abstract
Traditional treatments for type 1 and type 2 diabetes are often associated with side effects, including weight gain and hypoglycaemia that may offset the benefits of blood glucose lowering. The kidneys filter and reabsorb large amounts of glucose, and urine is almost free of glucose in normoglycaemia. The sodium-dependent glucose transporter (SGLT)-2 in the early proximal tubule reabsorbs the majority of filtered glucose. Remaining glucose is reabsorbed by SGLT1 in the late proximal tubule. Diabetes enhances renal glucose reabsorption by increasing the tubular glucose load and the expression of SGLT2 (as shown in mice), which maintains hyperglycaemia. Inhibitors of SGLT2 enhance urinary glucose excretion and thereby lower blood glucose levels in type 1 and type 2 diabetes. The load-dependent increase in SGLT1-mediated glucose reabsorption explains why SGLT2 inhibitors in normoglycaemic conditions enhance urinary glucose excretion to only ~50% of the filtered glucose. The role of SGLT1 in both renal and intestinal glucose reabsorption provides a rationale for the development of dual SGLT1/2 inhibitors. SGLT2 inhibitors lower blood glucose levels independent of insulin and induce pleiotropic actions that may be relevant in the context of lowering cardiovascular risk. Ongoing long-term clinical studies will determine whether SGLT2 inhibitors have a safety profile and exert cardiovascular benefits that are superior to traditional agents.
Keywords: Renal glucose reabsorption; anti-diabetic therapy; cardiovascular outcomes; kidney physiology.
© The Author(s) 2015.
Conflict of interest statement
Declaration of conflicting interests
V.V. was supported by investigator-initiated research grants from Bristol-Myers Squibb, AstraZeneca and Boehringer Ingelheim, Biberach. V.V. serves as a consultant for Boehringer Ingelheim and Janssen Pharmaceutical. E.M.W. serves on a Boehringer Ingelheim Advisory Board and has been supported by investigator-initiated research grants from Boehringer Ingelheim and Janssen Pharmaceuticals.
Figures
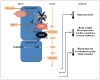
Figure 1
Glucose reabsorption in the renal proximal tubule. The basolateral Na+/K+ ATPase pumps Na+ out and K+ into the cell to establish an inward Na+ gradient. This gradient is used for Na+ and glucose co-transport across the luminal brush border of the early proximal tubule through SGLT2, and the glucose is passively returned via GLUT2 to the interstitium/bloodstream. In the late proximal tubule, SGLT1 is responsible for ‘mopping up’ remaining luminal glucose, while the role of basolateral GLUT1-facilitated glucose transport in this segment remains unclear. Apical efflux of K+ maintains the electrogenic gradient. Na+/K+ ATPase: sodium potassium adenosine triphosphatase active transporter; SGLT: sodium-dependent glucose transporter; GLUT: facilitative glucose transporter.
Figure 2
Downstream effects of proximal tubule SGLT1/2 inhibition. Glucose is freely filtered at the glomerulus into the tubular lumen, and normally, all of it is reabsorbed along the apical brush border membrane by the SGLT2 (and SGLT1 to a lesser extent) in the proximal tubule. Inhibition of SGLT2 or dual inhibition of SGLT1/2 establishes a concentration gradient that may drive glucose entry from the peritubular capillaries via the bidirectional GLUT2 transporter. The fate of glucose entering proximal tubule cells via this pathway is not known. SGLT inhibition reduces NHE3 activity and may contribute to increased luminal Na+ concentrations. NaCl detected at the macula densa and via tubuloglomerular feedback results in decreased single nephron glomerular filtration rate (SNGFR). Experimental evidence indicates that SGLT2 inhibition may inhibit the diabetes-induced upregulation of renal gluconeogenesis. Increased luminal glucose may facilitate intracellular urate exchange via GLUT9. This promotes uric acid urinary excretion reducing serum levels in diabetes. Diuresis and calorie loss result in reductions in body weight and blood pressure, translating into improved insulin sensitivity and reduced arterial stiffness. Finally, increased urinary excretion of glucose lowers hyperglycaemia and alleviates glucotoxicity on multiple tissue including benefits for insulin secretion. SGLT: sodium-dependent glucose transporter; GLUT: facilitative glucose transporter; NHE3: sodium hydrogen exchanger-3.
Similar articles
- Development of sotagliflozin, a dual sodium-dependent glucose transporter 1/2 inhibitor.
Lapuerta P, Zambrowicz B, Strumph P, Sands A. Lapuerta P, et al. Diab Vasc Dis Res. 2015 Mar;12(2):101-10. doi: 10.1177/1479164114563304. Diab Vasc Dis Res. 2015. PMID: 25690134 Review. - Sodium-glucose co-transporter inhibition in the treatment of diabetes: sweetening the pot.
Alvarez CA, Neeland IJ, McGuire DK. Alvarez CA, et al. Diab Vasc Dis Res. 2015 Mar;12(2):74-7. doi: 10.1177/1479164114563303. Diab Vasc Dis Res. 2015. PMID: 25690133 Free PMC article. No abstract available. - Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition.
Vallon V, Thomson SC. Vallon V, et al. Diabetologia. 2017 Feb;60(2):215-225. doi: 10.1007/s00125-016-4157-3. Epub 2016 Nov 22. Diabetologia. 2017. PMID: 27878313 Free PMC article. Review. - Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus.
Abdul-Ghani MA, Norton L, DeFronzo RA. Abdul-Ghani MA, et al. Am J Physiol Renal Physiol. 2015 Dec 1;309(11):F889-900. doi: 10.1152/ajprenal.00267.2015. Epub 2015 Sep 9. Am J Physiol Renal Physiol. 2015. PMID: 26354881 Free PMC article. Review. - SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials.
Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, Espadero RM, Woerle HJ, Broedl UC, Johansen OE. Inzucchi SE, et al. Diab Vasc Dis Res. 2015 Mar;12(2):90-100. doi: 10.1177/1479164114559852. Epub 2015 Jan 14. Diab Vasc Dis Res. 2015. PMID: 25589482 Free PMC article. Review.
Cited by
- Dapagliflozin targets SGLT2/SIRT1 signaling to attenuate the osteogenic transdifferentiation of vascular smooth muscle cells.
Li L, Liu H, Chai Q, Wei J, Qin Y, Yang J, Liu H, Qi J, Guo C, Lu Z. Li L, et al. Cell Mol Life Sci. 2024 Nov 9;81(1):448. doi: 10.1007/s00018-024-05486-8. Cell Mol Life Sci. 2024. PMID: 39520538 Free PMC article. - SGLT2 inhibitors and NLRP3 inflammasome: potential target in diabetic kidney disease.
Machado Júnior PAB, Lass A, Pilger BI, Fornazari R, Moraes TP, Pinho RA. Machado Júnior PAB, et al. J Bras Nefrol. 2024 Oct-Dec;46(4):e20230187. doi: 10.1590/2175-8239-JBN-2023-0187en. J Bras Nefrol. 2024. PMID: 39412512 Free PMC article. Review. - A self-reinforcing cycle hypothesis in heart failure pathogenesis.
Fernandez-Patron C, Lopaschuk GD, Hardy E. Fernandez-Patron C, et al. Nat Cardiovasc Res. 2024 Jun;3(6):627-636. doi: 10.1038/s44161-024-00480-6. Epub 2024 Jun 3. Nat Cardiovasc Res. 2024. PMID: 39196226 Review. - Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors and Cardiovascular Outcomes: A Review of Literature.
Mani S, Balasubramanian A, Veluswami K, Rao S, Aggarwal S. Mani S, et al. Cureus. 2024 Jul 4;16(7):e63796. doi: 10.7759/cureus.63796. eCollection 2024 Jul. Cureus. 2024. PMID: 39099905 Free PMC article. Review. - Effect of Jardiance on glucose uptake into astrocytomas.
Ghezzi C, Ellingson BM, Lai A, Liu J, Barrio JR, Wright EM. Ghezzi C, et al. J Neurooncol. 2024 Sep;169(2):437-444. doi: 10.1007/s11060-024-04746-8. Epub 2024 Jul 22. J Neurooncol. 2024. PMID: 39037688 Free PMC article.
References
- International Diabetes Federation. IDF diabetes Atlas. 6. Brussels: International Diabetes Federation; 2013.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. - PubMed
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK019567/DK/NIDDK NIH HHS/United States
- P30DK079337/DK/NIDDK NIH HHS/United States
- R01 DK056248/DK/NIDDK NIH HHS/United States
- R01DK56248/DK/NIDDK NIH HHS/United States
- R01DK19567/DK/NIDDK NIH HHS/United States
- P30 DK079337/DK/NIDDK NIH HHS/United States
- R01 HL094728/HL/NHLBI NIH HHS/United States
- R01HL094728/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical