Chronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder - PubMed (original) (raw)
Chronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder
Maria Razzoli et al. Front Nutr. 2015.
Abstract
Background: Eating disorders are associated with physical morbidity and appear to have causal factors like stressful life events and negative affect. Binge eating disorder (BED) is characterized by eating in a discrete period of time a larger than normal amount of food, a sense of lack of control over eating, and marked distress. There are still unmet needs for the identification of mechanisms regulating excessive eating, which is in part due to the lack of appropriate animal models. We developed a naturalistic murine model of subordination stress induced hyperphagia associated with the development of obesity. Here we tested the hypotheses that the eating responses of subordinate mice recapitulate the BED and that limiting hyperphagia could prevent stress-associated metabolic changes.
Methods: Adult male mice were exposed to a model of chronic subordination stress associated with the automated acquisition of food intake and we performed a detailed meal pattern analysis. Additionally, using a pair-feeding protocol was test the hypothesis that the manifestation of obesity and the metabolic syndrome could be prevented by limiting hyperphagia.
Results: The architecture of feeding of subordinate mice was disrupted during the stress protocol due to disproportionate amount of food ingested at higher rate and with shorter satiety ratio than control mice. Subordinate mice hyperphagia was further exacerbated in response to either hunger or to the acute application of a social defeat. Notably, the obese phenotype but not the fasting hyperglycemia of subordinate mice was abrogated by preventing hyperphagia in a pair feeding paradigm.
Conclusion: Overall these results support the validity of our chronic subordination stress to model binge eating disorder allowing for the determination of the underlying molecular mechanisms and the generation of testable predictions for innovative therapies, based on the understanding of the regulation and the control of food intake.
Keywords: ghrelin; glucose; meal pattern analysis; obesity; pair feeding.
Figures
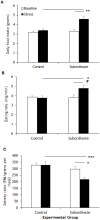
Figure 1
Meal-pattern analysis highlighted increased food intake in subordinate C57BL/6J mice (A) that was associated to a faster feeding rate (B) and to a shorter satiety ratio (C) than baseline and/or controls. Data represent group averages ± SEM. Control: N = 7; subordinate: N = 5. #p = 0.055, *p < 0.05, **p < 0.01, ***p < 0.001.
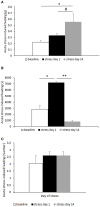
Figure 2
Hyperphagia was further exacerbated in subordinate CD1 mice in response to acute stress (social defeat for 10 min) in the subsequent 6 h (A) as well as to overnight fasting followed by refeeding (B). Data represent group averages ± SEM. (A) Control: N = 15; subordinate: N = 13. (B) Control: N = 15; subordinate: N = 10. *p < 0.05, ***p < 0.001.
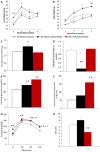
Figure 3
Meal-pattern and time-course analysis of acute stress-induced hyperphagia in C57BL/6J mice. Meal intake was increased over time in subordinate (A), while meal duration (B) was initially increased to later on diminish in correspondence with a meal frequency that remained stable over time (C). Data represent group averages ± SEM. Control: N = 7; subordinate: N = 5. #p = 0.055, *p < 0.05, **p < 0.01.
Figure 4
Pair-feeding subordinate mice prevents stress-induced vulnerability to diet-induced obesity. (A) Food intake, data are presented as least square means ± SEM; the covariate is the baseline food intake, average = 22.5 kcal [F(1,49) = 22.24, p < 0.01; ad libitum fed control: N = 12; ad libitum fed subordinate: N = 28; pair fed subordinate: N = 13]; (B) body weight gain, data are presented as least square means ± SEM; the covariate is the baseline body weight, average = 41.2 g [F(1,55) = 198.37, p < 0.001; ad libitum fed control: N = 22; ad libitum fed subordinate: N = 34; pair fed subordinate: N = 13]; (C) perigonadal white adipose tissue (WAT) [ad libitum fed control: N = 12; ad libitum fed subordinate: N = 26; pair fed subordinate: N = 12]; (D) total ghrelin [ad libitum fed control: N = 5; ad libitum fed subordinate: N = 6; pair fed subordinate: N = 8]; (E) glucose [ad libitum fed control: N = 21; ad libitum fed subordinate: N = 26; pair fed subordinate: N = 9]; (F) corticosterone [ad libitum fed control: N = 17; ad libitum fed subordinate: N = 23; pair fed subordinate: N = 8]; (G,H) glucose tolerance test [ad libitum fed control: N = 16; ad libitum fed subordinate: N = 19; pair fed subordinate: N = 13]. (D–H) Data represent group averages ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 vs. control. In (G), ** refer to binary comparisons between pair fed subordinate and control mice, while ++ refer to binary comparisons between ad libitum fed subordinate and control mice.
Similar articles
- Stress, overeating, and obesity: Insights from human studies and preclinical models.
Razzoli M, Pearson C, Crow S, Bartolomucci A. Razzoli M, et al. Neurosci Biobehav Rev. 2017 May;76(Pt A):154-162. doi: 10.1016/j.neubiorev.2017.01.026. Epub 2017 Mar 11. Neurosci Biobehav Rev. 2017. PMID: 28292531 Free PMC article. Review. - Vulnerability to chronic subordination stress-induced depression-like disorders in adult 129SvEv male mice.
Dadomo H, Sanghez V, Di Cristo L, Lori A, Ceresini G, Malinge I, Parmigiani S, Palanza P, Sheardown M, Bartolomucci A. Dadomo H, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Aug 1;35(6):1461-71. doi: 10.1016/j.pnpbp.2010.11.016. Epub 2010 Nov 17. Prog Neuropsychopharmacol Biol Psychiatry. 2011. PMID: 21093519 - Stress-induced eating in women with binge-eating disorder and obesity.
Klatzkin RR, Gaffney S, Cyrus K, Bigus E, Brownley KA. Klatzkin RR, et al. Biol Psychol. 2018 Jan;131:96-106. doi: 10.1016/j.biopsycho.2016.11.002. Epub 2016 Nov 9. Biol Psychol. 2018. PMID: 27836626 - A new animal model of binge eating: key synergistic role of past caloric restriction and stress.
Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. Hagan MM, et al. Physiol Behav. 2002 Sep;77(1):45-54. doi: 10.1016/s0031-9384(02)00809-0. Physiol Behav. 2002. PMID: 12213501 - The Role of the Endocannabinoid System in Binge Eating Disorder.
Bourdy R, Befort K. Bourdy R, et al. Int J Mol Sci. 2023 May 31;24(11):9574. doi: 10.3390/ijms24119574. Int J Mol Sci. 2023. PMID: 37298525 Free PMC article. Review.
Cited by
- Stress-driven potentiation of lateral hypothalamic synapses onto ventral tegmental area dopamine neurons causes increased consumption of palatable food.
Linders LE, Patrikiou L, Soiza-Reilly M, Schut EHS, van Schaffelaar BF, Böger L, Wolterink-Donselaar IG, Luijendijk MCM, Adan RAH, Meye FJ. Linders LE, et al. Nat Commun. 2022 Nov 12;13(1):6898. doi: 10.1038/s41467-022-34625-7. Nat Commun. 2022. PMID: 36371405 Free PMC article. - Relationship between Orexigenic Peptide Ghrelin Signal, Gender Difference and Disease.
Yamada C. Yamada C. Int J Mol Sci. 2021 Apr 5;22(7):3763. doi: 10.3390/ijms22073763. Int J Mol Sci. 2021. PMID: 33916403 Free PMC article. Review. - The Good, the Bad and the Unknown Aspects of Ghrelin in Stress Coping and Stress-Related Psychiatric Disorders.
Fritz EM, Singewald N, De Bundel D. Fritz EM, et al. Front Synaptic Neurosci. 2020 Oct 27;12:594484. doi: 10.3389/fnsyn.2020.594484. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 33192444 Free PMC article. Review. - Assessing the effects of stress on feeding behaviors in laboratory mice.
Francois M, Canal Delgado I, Shargorodsky N, Leu CS, Zeltser L. Francois M, et al. Elife. 2022 Feb 15;11:e70271. doi: 10.7554/eLife.70271. Elife. 2022. PMID: 35167441 Free PMC article.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (Vol. 5). Arlington, VA: American Psychiatric Publishing; (2013).
LinkOut - more resources
Full Text Sources
Other Literature Sources