Altered Cholesterol Intracellular Trafficking and the Development of Pathological Hallmarks of Sporadic AD - PubMed (original) (raw)
Altered Cholesterol Intracellular Trafficking and the Development of Pathological Hallmarks of Sporadic AD
Xuesong Chen et al. J Parkinsons Dis Alzheimers Dis. 2014.
Abstract
Compared to the rare familial early onset Alzheimer's disease (AD) that results from gene mutations in AbPP and presenilin-1, the pathogenesis of sporadic AD is much more complex and is believed to result from complex interactions between nutritional, environmental, epigenetic and genetic factors. Among those factors, the presence APOE4 is still the single strongest genetic risk factor for sporadic AD. However, the exact underlying mechanism whereby apoE4 contributes to the pathogenesis of sporadic AD remains unclear. Here, we discuss how altered cholesterol intracellular trafficking as a result of apoE4 might contribute to the development of pathological hallmarks of AD including brain deposition of amyloid beta (Ab), neurofibrillary tangles, and synaptic dysfunction.
Keywords: Alzheimer’s disease; ApoE4; Cholesterol; Niemann-Pick type C disease.
Figures
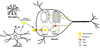
Figure 1. Cholesterol homeostasis in brain
Brain cholesterol is almost completely dependent on in situ synthesis of HDL-like apoE-cholesterol by astrocytes. Such HDL-like apoE-cholesterol supplies the neuronal need of cholesterol via receptor-mediated endocytosis, a process where apoE-cholesterol bound to their receptors are internalized, transported to endolysosomes, hydrolyzed to free cholesterol, and from where free cholesterol is transported to various intracellular compartments (ER, Golgi) or plasma membrane via a mechanism involving the Niemann-Pick type C proteins type-1 and -2 proteins.
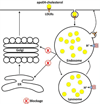
Figure 2. ApoE4-induced cholesterol dyshomeostasis
ApoE-cholesterol is up-taken by neurons via receptor-mediated endocytosis with the assistance of LDLRs. Different apoE isoforms have different affinities for lipids and receptors for cholesterol uptake, and the associations between cholesterol and different apoE can result in drastic differences in endocytic trafficking and distribution of cholesterol in neurons. ApoE4 could lead to impaired recycling of cholesterol back to ER, Golgi and plasma membranes, where cholesterol is needed for membrane repair, neurite outgrowth, and synaptic plasticity. In addition, apoE4 could increase accumulation of cholesterol in endolysosomes thus disturbing endolysosome function.
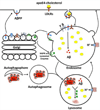
Figure 3. ApoE4-cholesterol contributes to the development of AD pathology
ApoE4 could promote AβPP internalization or impair recycling of internalized AβPP, and thus to leads enhanced amyloidgenic processing of AβPP in endosome, the site where BACE-1 and γ-secretase are almost exclusively located and active in the acidic environment. Another mechanism whereby apoE4 promotes Aβ generation might result from apoE4-induced endolysosome cholesterol accumulation and endolysosome dysfunction, which could enhance Aβ generation in endosomes and inhibit Aβ degradation in lysosomes. Because hyperphosphorylated tau can be degraded in autophagosomes-lysosomes, apoE4-induced cholesterol accumulation in endolysosome and subsequent endolysosome dysfunction could impair tau degradation in autophagosome-lysosomes, thus leading to increased accumulation of hyperphosphorylated tau and the development of neurofibrillary tangle.
Similar articles
- Apolipoprotein gene and its interaction with the environmentally driven risk factors: molecular, genetic and epidemiological studies of Alzheimer's disease.
Lahiri DK, Sambamurti K, Bennett DA. Lahiri DK, et al. Neurobiol Aging. 2004 May-Jun;25(5):651-60. doi: 10.1016/j.neurobiolaging.2003.12.024. Neurobiol Aging. 2004. PMID: 15172744 - Role of LDL cholesterol and endolysosomes in amyloidogenesis and Alzheimer's disease.
Chen X, Hui L, Geiger JD. Chen X, et al. J Neurol Neurophysiol. 2014 Oct 1;5(5):236. doi: 10.4172/2155-9562.1000236. J Neurol Neurophysiol. 2014. PMID: 26413387 Free PMC article. - New insight into the role of altered brain cholesterol metabolism in the pathogenesis of AD: A unifying cholesterol hypothesis and new therapeutic approach for AD.
Yang X, Yao K, Zhang M, Zhang W, Zu H. Yang X, et al. Brain Res Bull. 2025 May;224:111321. doi: 10.1016/j.brainresbull.2025.111321. Epub 2025 Mar 29. Brain Res Bull. 2025. PMID: 40164234 Review. - Streptozotocin Intracerebroventricular-Induced Neurotoxicity and Brain Insulin Resistance: a Therapeutic Intervention for Treatment of Sporadic Alzheimer's Disease (sAD)-Like Pathology.
Kamat PK, Kalani A, Rai S, Tota SK, Kumar A, Ahmad AS. Kamat PK, et al. Mol Neurobiol. 2016 Sep;53(7):4548-62. doi: 10.1007/s12035-015-9384-y. Epub 2015 Aug 23. Mol Neurobiol. 2016. PMID: 26298663 Review. - Intracellular Trafficking Mechanisms of Synaptic Dysfunction in Alzheimer's Disease.
Perdigão C, Barata MA, Araújo MN, Mirfakhar FS, Castanheira J, Guimas Almeida C. Perdigão C, et al. Front Cell Neurosci. 2020 Apr 17;14:72. doi: 10.3389/fncel.2020.00072. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32362813 Free PMC article.
Cited by
- Non-alcoholic fatty liver disease induces signs of Alzheimer's disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model.
Kim DG, Krenz A, Toussaint LE, Maurer KJ, Robinson SA, Yan A, Torres L, Bynoe MS. Kim DG, et al. J Neuroinflammation. 2016 Jan 5;13:1. doi: 10.1186/s12974-015-0467-5. J Neuroinflammation. 2016. PMID: 26728181 Free PMC article. - Astrocytic Propagation of Tau in the Context of Alzheimer's Disease.
Fleeman RM, Proctor EA. Fleeman RM, et al. Front Cell Neurosci. 2021 Mar 17;15:645233. doi: 10.3389/fncel.2021.645233. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33815065 Free PMC article. Review. - Towards a Unitary Hypothesis of Alzheimer's Disease Pathogenesis.
Area-Gomez E, Schon EA. Area-Gomez E, et al. J Alzheimers Dis. 2024;98(4):1243-1275. doi: 10.3233/JAD-231318. J Alzheimers Dis. 2024. PMID: 38578892 Free PMC article. - Amyloid-beta Alzheimer targets - protein processing, lipid rafts, and amyloid-beta pores.
Arbor SC, LaFontaine M, Cumbay M. Arbor SC, et al. Yale J Biol Med. 2016 Mar 24;89(1):5-21. eCollection 2016 Mar. Yale J Biol Med. 2016. PMID: 27505013 Free PMC article. Review.
References
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
- Goate A, Hardy J. Twenty years of Alzheimer’s disease-causing mutations. J Neurochem. 2011;120(Suppl 1):3–8. - PubMed
Grants and funding
- P30 GM103329/GM/NIGMS NIH HHS/United States
- R01 MH100972/MH/NIMH NIH HHS/United States
- R01 MH105329/MH/NIMH NIH HHS/United States
- R01 NS065957/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources