Pluripotent stem cells reveal erythroid-specific activities of the GATA1 N-terminus - PubMed (original) (raw)
. 2015 Mar 2;125(3):993-1005.
doi: 10.1172/JCI75714. Epub 2015 Jan 26.
Daniel VanDorn, Amy E Campbell, Marisol Betensky, Philip R Arca, Yu Yao, Paul Gadue, Fernando F Costa, Richard L Nemiroff, Gerd A Blobel, Deborah L French, Ross C Hardison, Mitchell J Weiss, Stella T Chou
- PMID: 25621499
- PMCID: PMC4362246
- DOI: 10.1172/JCI75714
Pluripotent stem cells reveal erythroid-specific activities of the GATA1 N-terminus
Marta Byrska-Bishop et al. J Clin Invest. 2015.
Abstract
Germline GATA1 mutations that result in the production of an amino-truncated protein termed GATA1s (where s indicates short) cause congenital hypoplastic anemia. In patients with trisomy 21, similar somatic GATA1s-producing mutations promote transient myeloproliferative disease and acute megakaryoblastic leukemia. Here, we demonstrate that induced pluripotent stem cells (iPSCs) from patients with GATA1-truncating mutations exhibit impaired erythroid potential, but enhanced megakaryopoiesis and myelopoiesis, recapitulating the major phenotypes of the associated diseases. Similarly, in developmentally arrested GATA1-deficient murine megakaryocyte-erythroid progenitors derived from murine embryonic stem cells (ESCs), expression of GATA1s promoted megakaryopoiesis, but not erythropoiesis. Transcriptome analysis revealed a selective deficiency in the ability of GATA1s to activate erythroid-specific genes within populations of hematopoietic progenitors. Although its DNA-binding domain was intact, chromatin immunoprecipitation studies showed that GATA1s binding at specific erythroid regulatory regions was impaired, while binding at many nonerythroid sites, including megakaryocytic and myeloid target genes, was normal. Together, these observations indicate that lineage-specific GATA1 cofactor associations are essential for normal chromatin occupancy and provide mechanistic insights into how GATA1s mutations cause human disease. More broadly, our studies underscore the value of ESCs and iPSCs to recapitulate and study disease phenotypes.
Figures
Figure 7. Loss of the N-terminus selectively inhibits GATA1 binding to erythroid genes in Gata1– MEPs.
(A) Flow cytometry of G1ME cells at 42 and 96 hours after transduction with HA-GATA1fl or HA-GATA1s. Numbers denote percentage of total cells in the indicated quadrant. (B) May-Grünwald-Giemsa staining of G1ME-derived erythroblasts (left) and megakaryocytes (right) 96 hours after transduction. Scale bars: 20 μm. (C) Western blot at 42 hours after transduction showing expression of HA-GATA1fl and HA-GATA1s relative to β-actin. ND, not determined. (D) Genome-wide ChIP-seq binding signals of GATA1fl and GATA1s at 42 hours after transduction. Plotted are mean read counts (n = 2 replicates each). non-DB, nondifferentially bound (gray dots); DB, differentially bound sites (blue and cyan; FDR < 0.1). (E) Functional enrichment analysis using GREAT. Plotted are significance values for top 10 mouse phenotype and GO biological process enrichment terms, classified as erythroid, megakaryocytic, myeloid, other hematopoietic, or cardiovascular and other. List of terms can be found in Supplemental Table 5. (F) GATA1 binding at the β-globin locus. ChIP-seq tracks, top to bottom: GATA1fl and GATA1s in G1MEs, GATA1fl in primary mouse erythroblasts and megakaryocytes (42). Rectangles above tracks: size of binding sites analyzed in differential binding analysis; gray, non-DB; blue, DB. (G) Anti-HA ChIP at selected GATA1-binding sites in G1MEs shown as ratio of GATA1s to GATA1fl occupancy ± SEM (n = 4). *Ratio significantly different than 1 at P < 0.05 (2-tailed Student’s t test). IgG GATA1s and IgG GATA1fl controls for nonspecific binding.
Figure 6. Single-cell gene expression analysis predicts erythroid-to-myeloid fate bias in GATA1s mutant progenitors.
(A) Projections of expression patterns of iPSC-derived committed cells (filled circles in both plots) and CD43+CD41+CD235+ progenitors expressing WT GATA1 (left, open diamonds) or GATA1s (right, open diamonds) onto linear discriminants 1 and 2 (LD1 and LD2). Projections for each cell are colored according to the classification obtained from LDA using a probability threshold of greater than 0.90. Classifications of committed cells: erythroid (red circles), megakaryocytic (blue circles), myeloid (green circles); classifications of WT GATA1 (left) or GATA1s (right) progenitors: predicted erythroid (pEry) (red diamonds), predicted megakaryocytic (pMeg) (blue diamonds), predicted myeloid (pMyelo) (green diamonds). Gray diamonds represent progenitors that were unclassified at a probability threshold of greater than 0.90. (B) Hierarchical clustering on probabilities of belonging to erythroid, megakaryocytic, or myeloid class assigned by LDA to progenitor cells. WT GATA1 or GATA1s progenitors that were assigned to a given class with a probability of greater than 0.90 are represented on 2 heat maps on the left, while cells that were unclassified (probability < 0.90) are shown on 2 heat maps on the right. (C) Classification of CD43+CD41+CD235+ progenitors using LDA. Plotted are fractions of WT GATA1 and GATA1s progenitor populations (consisting of a total of 311 and 274 single cells, respectively) classified into predicted cell fate with a probability of greater than 0.90. The remaining cells are plotted as unclassified. *P < 0.05 (Fisher’s exact test).
Figure 5. PCA identifies lineage signatures and reveals heterogeneity within progenitor cell populations.
Single cells within CD43+CD41+CD235+ hematopoietic progenitor populations derived from WT and mutant iPSCs were purified by flow cytometry and analyzed for gene expression by qPCR. For comparison, committed erythroid (CD41–CD235+), myeloid (CD45+CD18+), and megakaryocytic (CD41+CD42+) cells generated from WT iPSCs were purified and analyzed in parallel. (A) Numbers of analyzed single cells of each genotype (total 755 single cells). WT lineage committed cells: myeloid (myelo), megakaryocytic, and erythroid (ery). CD43+CD41+CD235+ progenitor genotypes: euploid/WT GATA1, euploid/GATA1s, T21/WT GATA1, T21/GATA1s. (B) PCA on 170 committed erythroid (red), myeloid (green), and megakaryocytic (blue) cells based on expression patterns of 94 genes (right plot). PC loadings obtained from this analysis (right) were used to project 274 GATA1s (purple) and 311 WT GATA1 (black) progenitor cells onto PC1 and PC2 (left). (C) Smooth kernel density estimate of PC1 scores of GATA1s (purple line) and WT GATA1 progenitors (black line). Purple and black vertical lines represent mean PC1 scores. Shaded areas around the mean correspond to 90% confidence interval for the mean. *P = 10–13 (Mann-Whitney U test). (D) Violin plots showing distributions of single-cell expression levels of 3 genes that were upregulated (left) and 3 genes that were downregulated (right) in GATA1s vs. WT GATA1 progenitors in 5 cell types analyzed (FDR < 0.05; Mann-Whitney U test followed by BH-FDR correction). lfc, lfc of mean expression between GATA1s and WT GATA1 progenitors.
Figure 4. Global transcriptome analysis demonstrates that GATA1s mutations downregulate erythroid and upregulate myelo-megakaryocytic genes.
(A) Mean expression values of 10,873 expressed genes in T21/GATA1s (n = 3 replicates) versus T21/WT GATA1 (n = 6 replicates) iPSC–derived CD43+CD41+CD235+ progenitors. 273 genes were differentially expressed between T21/GATA1s and T21/WT GATA1, with a fold change of mean expression of less than 2 (217 genes, blue) or 2-fold or greater (56 genes, green). (B) GSEA on 273 differentially expressed genes using erythroid, megakaryocytic, and myeloid expression profiles from Novershtern et al. (30). Top: enrichment of 154 genes that were upregulated in T21/GATA1s as compared with T21/WT GATA1 progenitors in myeloid versus erythroid signature genes and in megakaryocytic versus erythroid genes. Bottom: enrichment of 119 downregulated genes in erythroid versus myeloid genes and in erythroid versus megakaryocytic genes. NES, normalized enrichment score; P values shown are from modified Kolmogorov-Smirnov test as implemented in GSEA. (C) Heat maps showing expression levels of 2-fold or greater upregulated (top) and downregulated (bottom) genes in T21/GATA1s versus T21/WT GATA1 progenitors (left) as well as in lineage-committed cells (right) based on expression levels in erythroid (n = 7 replicates of CD34–CD71loGlyA+, 6 of CD34–CD71–GlyA+ cells), myeloid (n = 6 replicates of basophils, 5 of eosinophils, 4 of neutrophils), and megakaryocytic (n = 5 replicates of CFU-megakaryocytes, CD34+CD41+CD61+CD45–, 7 of mature megakaryocytes, CD34–CD41+CD61+CD45–) cells from Novershtern et al. (30). Color scheme is row normalized from blue to red corresponding to minimum to maximum expression values in a given row, respectively. ZC3H12C, COL24A1, MIR221, P2RY12, PARP9, and OCIAD2 were not represented on microarrays from Novershtern et al. (30).
Figure 3. Dose-dependent restoration of erythropoiesis with enforced expression of full‑length GATA1 and truncated GATA1s.
T21/WT GATA1 and T21/GATA1s iPSC–derived CD43+CD41+CD235+ progenitors were transduced with lentivirus containing vector alone or encoding GATA1fl or GATA1s and cultured with EPO and SCF (n = 4 replicates). (A) Representative flow-cytometric analysis after 6 days of culture. Numbers denote percentage of total cells in the indicated gate. (B) Average percentage of CD235+ erythroblasts in transduced (GFP+) cells after 6 days of culture. (C) PCR showing relative GATA1 mRNA expression 2 days after lentiviral infection with control vector, GATA1fl, or GATA1s. The genotype of progenitors is shown on the x axis. (D) Expression of selected erythroid mRNAs in flow-cytometry–purified CD235+ infected (GFP+) cells.
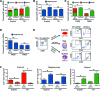
Figure 2. GATA1s mutations impair erythropoiesis and enhance the production of myeloid cells and megakaryocytes in iPSC-derived CD43+CD41+CD235+ progenitors.
(A) Methylcellulose colony assays containing SCF, IL-3, EPO, GM-CSF, and (B) colony-forming megakaryocyte (CFU-Mk) assays with TPO, IL-3, and IL-6 of CD43+CD41+CD235+ progenitors. Results show mean values ± SEM of all lines performed in triplicate (n = 4 euploid/WT GATA1, 2 euploid/GATA1s, 4 T21/WT GATA1, and 5 T21/GATA1s lines). (C) Methylcellulose colony assays and (D) CFU-Mk formation from CD43+CD41+CD235+ progenitors from 2 pairs of isogenic iPSC clones with the indicated GATA1 alleles, performed as in A and B. (E) Schematic and representative flow-cytometry analysis of WT GATA1 and GATA1s CD43+CD41+CD235+ progenitors grown in liquid culture to preferentially support erythroid (CD71+CD235+), megakaryocyte (CD41+CD42+), and myeloid (CD45+CD18+) differentiation. Numbers denote percentage of total cells in the indicated gate. (F) Fold expansion of erythroid, megakaryocyte, and myeloid cells after 6 days in lineage-specific liquid cultures of euploid or T21 progenitors expressing WT GATA1 or GATA1s (n = 4 independent assays for each bar with the exception of n = 3 for euploid/GATA1s in erythroid culture, n = 5 for T21/WT GATA1 in erythroid culture, and n = 5 for T21/WT GATA1 and T21/GATA1s in megakaryocyte culture). *P < 0.05 (2-tailed Student’s t test).
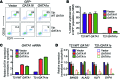
Figure 1. GATA1s mutations inhibit erythropoiesis from patient-derived iPSCs.
(A) Flow-cytometry analysis of CD34+/–CD43+CD41+CD235+ progenitors within total EB cultures on day 7 of hematopoietic differentiation and (B) suspension cells released from EBs on day 12 showing mature hematopoietic lineages: erythroid (CD41–CD235+), megakaryocytic (Meg, CD41+CD42+), and myeloid (CD45+CD18+). Numbers denote percentage of total cells in the indicated gate. (C) Frequency of CD43+CD41+CD235+ progenitor cells in EB cultures on days 7 and 8 of hematopoietic differentiation (n = 6; 17 independent experiments for euploid and T21 groups, respectively). (D) Summary of distribution of lineage-committed cells in EB suspension cultures on differentiation day 12 (n = 12; 20 independent assays for euploid and T21 groups, respectively). (E) Hematopoietic cell morphology on day 20 of differentiation cultures of isogenic WT GATA1 or GATA1s iPSCs. Scale bars: 50 μm. (F) Western blot of iPSC-derived hematopoietic cells. (G) DNA sequence analysis showing WT GATA1 and a heterozygous exon 2 mutation in 2 different iPSC clones from a female with DS and TMD. (H) Isogenic lines from 2 different TMD patients were analyzed. Percentages of mature lineages from day-12 EB suspension cultures, as in D. (n = 6; 4 independent assays for TMD8 and TMD9, respectively). *P < 0.005 for myeloid and erythroid lineages (2-tailed Student’s t test).
Similar articles
- GATA1 in Normal and Pathologic Megakaryopoiesis and Platelet Development.
Takasaki K, Chou ST. Takasaki K, et al. Adv Exp Med Biol. 2024;1459:261-287. doi: 10.1007/978-3-031-62731-6_12. Adv Exp Med Biol. 2024. PMID: 39017848 Review. - Global transcriptome and chromatin occupancy analysis reveal the short isoform of GATA1 is deficient for erythroid specification and gene expression.
Chlon TM, McNulty M, Goldenson B, Rosinski A, Crispino JD. Chlon TM, et al. Haematologica. 2015 May;100(5):575-84. doi: 10.3324/haematol.2014.112714. Epub 2015 Feb 14. Haematologica. 2015. PMID: 25682601 Free PMC article. - Chromatin occupancy and epigenetic analysis reveal new insights into the function of the GATA1 N terminus in erythropoiesis.
Ling T, Birger Y, Stankiewicz MJ, Ben-Haim N, Kalisky T, Rein A, Kugler E, Chen W, Fu C, Zhang K, Patel H, Sikora JW, Goo YA, Kelleher N, Zou L, Izraeli S, Crispino JD. Ling T, et al. Blood. 2019 Nov 7;134(19):1619-1631. doi: 10.1182/blood.2019001234. Blood. 2019. PMID: 31409672 Free PMC article. - Modeling Down Syndrome Myeloid Leukemia by Sequential Introduction of GATA1 and STAG2 Mutations in Induced Pluripotent Stem Cells with Trisomy 21.
Barwe SP, Sebastian A, Sidhu I, Kolb EA, Gopalakrishnapillai A. Barwe SP, et al. Cells. 2022 Feb 11;11(4):628. doi: 10.3390/cells11040628. Cells. 2022. PMID: 35203280 Free PMC article. - Regulation of GATA1 levels in erythropoiesis.
Gutiérrez L, Caballero N, Fernández-Calleja L, Karkoulia E, Strouboulis J. Gutiérrez L, et al. IUBMB Life. 2020 Jan;72(1):89-105. doi: 10.1002/iub.2192. Epub 2019 Nov 25. IUBMB Life. 2020. PMID: 31769197 Review.
Cited by
- Analysis of microisolated frontal cortex excitatory layer III and V pyramidal neurons reveals a neurodegenerative phenotype in individuals with Down syndrome.
Alldred MJ, Pidikiti H, Ibrahim KW, Lee SH, Heguy A, Hoffman GE, Roussos P, Wisniewski T, Wegiel J, Stutzmann GE, Mufson EJ, Ginsberg SD. Alldred MJ, et al. Acta Neuropathol. 2024 Aug 6;148(1):16. doi: 10.1007/s00401-024-02768-0. Acta Neuropathol. 2024. PMID: 39105932 - GATA1 in Normal and Pathologic Megakaryopoiesis and Platelet Development.
Takasaki K, Chou ST. Takasaki K, et al. Adv Exp Med Biol. 2024;1459:261-287. doi: 10.1007/978-3-031-62731-6_12. Adv Exp Med Biol. 2024. PMID: 39017848 Review. - Modeling primitive and definitive erythropoiesis with induced pluripotent stem cells.
Pavani G, Klein JG, Nations CC, Sussman JH, Tan K, An HH, Abdulmalik O, Thom CS, Gearhart PA, Willett CM, Maguire JA, Chou ST, French DL, Gadue P. Pavani G, et al. Blood Adv. 2024 Mar 26;8(6):1449-1463. doi: 10.1182/bloodadvances.2023011708. Blood Adv. 2024. PMID: 38290102 Free PMC article. - Inherent genome instability underlies trisomy 21-associated myeloid malignancies.
Chen CC, Silberman RE, Ma D, Perry JA, Khalid D, Pikman Y, Amon A, Hemann MT, Rowe RG. Chen CC, et al. Leukemia. 2024 Mar;38(3):521-529. doi: 10.1038/s41375-024-02151-8. Epub 2024 Jan 20. Leukemia. 2024. PMID: 38245602 - Ginsenoside Rd Induces Differentiation of Myeloid Leukemia Cells via Regulating ERK/GSK-3β Signaling Pathway.
Jiang YX, Zhao YN, Yu XL, Yin LM. Jiang YX, et al. Chin J Integr Med. 2024 Jul;30(7):588-599. doi: 10.1007/s11655-023-3561-z. Epub 2023 Dec 12. Chin J Integr Med. 2024. PMID: 38085388
References
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL099656/HL/NHLBI NIH HHS/United States
- P30 DK090969/DK/NIDDK NIH HHS/United States
- R56 DK065806/DK/NIDDK NIH HHS/United States
- T32 HL007150/HL/NHLBI NIH HHS/United States
- F32 HL010166/HL/NHLBI NIH HHS/United States
- R01 DK065806/DK/NIDDK NIH HHS/United States
- U54 HD086984/HD/NICHD NIH HHS/United States
- U01 HL099993/HL/NHLBI NIH HHS/United States
- K08 HL093290/HL/NHLBI NIH HHS/United States
- R01 DK100854/DK/NIDDK NIH HHS/United States
- RC2 HL10166/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases