Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques - PubMed (original) (raw)
Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques
Carlo Condello et al. Nat Commun. 2015.
Abstract
In Alzheimer's disease (AD), β-amyloid (Aβ) plaques are tightly enveloped by microglia processes, but the significance of this phenomenon is unknown. Here we show that microglia constitute a barrier with profound impact on plaque composition and toxicity. Using high-resolution confocal and in vivo two-photon imaging in AD mouse models, we demonstrate that this barrier prevents outward plaque expansion and leads to compact plaque microregions with low Aβ42 affinity. Areas uncovered by microglia are less compact but have high Aβ42 affinity, leading to the formation of protofibrillar Aβ42 hotspots that are associated with more severe axonal dystrophy. In ageing, microglia coverage is reduced leading to enlarged protofibrillar Aβ42 hotspots and more severe neuritic dystrophy. CX3CR1 gene deletion or anti-Aβ immunotherapy causes expansion of microglia coverage and reduced neuritic dystrophy. Failure of the microglia barrier and the accumulation of neurotoxic protofibrillar Aβ hotspots may constitute novel therapeutic and clinical imaging targets for AD.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures
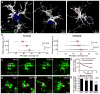
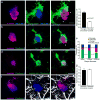
Figure 1. Microglia process envelopment of plaques is stable over weeks in vivo
(a) Representative confocal images of Iba1 immunolabeled microglia around Thioflavin S-labeled amyloid plaques in a 4-month-old 5xFAD mouse. (b) Quantification of microglia coverage in 4-month-old 5xFAD and 4-month-old CRND8 mice. Microglia coverage was quantified as the percentage of plaque perimeter contacted by microglia process. N>90 plaques (1–20 μm in diameter) from 5 animals for each transgenic line. Red bars represent mean ± s.e.m. (unpaired t test, t=(84)2.998 and (156)2.422 in 5xFAD and t=(218)2.379 and (362)2.506 in CRND8). (c) In vivo time-lapse images of microglia around amyloid plaques in _CX3CR1_-GFP x 5xFAD mouse. Blue arrows: microglia processes contacting amyloid plaques; red arrows: processes not in contact with amyloid plaque and extended between 20th and 30th minutes of the imaging session; red arrow-heads: processes not in contact with amyloid plaque and regressed between 20th and 30th minutes of the imaging session. (d) Quantification of microglia process stability over 30 minutes. N=15 plaques in 5 animals. Data represent mean s.e.m.. (e and f). In vivo time-lapse imaging over weeks to months reveals the stability of microglia processes contacting amyloid plaques. Stable processes: yellow arrows; newly added processes: red arrows; regressed processes: blue arrows. (g) Quantification of long-term dynamism of microglia processes contacting amyloid plaques. N=51 plaques in 9 animals. Scale bars: 5μm in c, e and f.
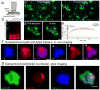
Figure 2. In vivo brain infusion reveals hotspots of Aβ42 binding to existing plaques
(a) Hylite-555 labeled Aβ40 or Aβ42 was infused into the mouse brain via the subarachnoid space and imaged through a thinned-skull window away from the injection site. (b) SDS-PAGE analysis of soluble Aβ42 used for in vivo infusion and ex vivo brain slice labeling. Fluorescently-labeled Aβ42 stock solution (DMSO, 1μg/μl) was diluted in ACSF (1:9 v/v) on ice. Three different preparations of Aβ42 were analyzed using SDS-PAGE gel. The SDS-PAGE analysis indicated that the main Aβ species injected were monomers and small oligomers. (c and d) In vivo two-photon time-lapse images in CX3CR1/5XFAD mice show that Aβ42-555 (red) rapidly binds to existing amyloid plaques, pre-labeled by FSB (blue) that are extensively surrounded by microglia (green). (e) Quantification of Aβ42-555 fluorescence during injection. Aβ42-555 binds to pre-existing amyloid plaque rapidly and with very high specificity. Internalization by surrounding microglia was undetectable in this time-frame. N=30 plaques in 5 animals. Data represents mean ± s.e.m.. (f) High magnification two-photon images show hotspots of Aβ42-555 binding to amyloid plaques (arrowhead). In contrast Aβ40-555 infusions led to homogeneous binding to amyloid plaques. (g) Simultaneous in vivo infusion of Aβ42-555 and Aβ40-488 followed by confocal imaging in fixed slices.
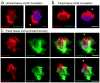
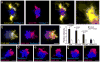
Figure 3. Endogenous Aβ42 accumulates within plaque hotspots in vivo
(a and b) Representative confocal images show hotspots of Aβ42-555 binding (arrowheads) in fresh unfixed (a) and fixed-permeabilized brain slices (b). (c) Immunohistochemistry with Aβ42 and Aβ40 c-terminus specific antibodies on fixed brain slices from mice that did not receive an Aβ-555 injections. Scale bars: 5μm in all panels.
Figure 4. Aβ42 hotspots occur in distinct plaque regions with sparse fibrillar amyloid
(a) Representative confocal images of Aβ42-555 binding to fibrillar amyloid plaque (asterisk indicates a prominent Aβ42 hot-spot). Scale bar: 5μm. Dashed squares point to areas with (green) and without (orange) an Aβ42 hotspot. (b, c) Intensity (heat) maps of plaque borders pointing towards a hotspot (b) and a non-hotspot (c) area, depicted at low zoom in (a). The magenta outline in b and c represents the thresholded plaque border separating the plaque core from the halo. (d,e) Line profiles of fluorescence intensities across the plaque borders (magenta dotted lines) as shown in b and c. Data represents mean ± s.e.m. (f–h) Quantification of thioflavin S fluorescence change as a function of distance from the plaque center in hotspot and non-hotspot areas. The slope of the curves indicates the rate of fluorescence change (how compact are the fibrils at the plaque edge) and the area under the curve represents the amount of fibrillar amyloid (total TS fluorescence) in the plaque halo area. Data represents mean ± s.e.m. (unpaired t-test, t=(47)4.005 in (g), t=(34)4.299 in (h)). For (d–h) N=30 plaques with diameter less than 10μm from 2 5xFAD mice (6-month-old).
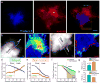
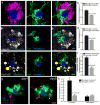
Figure 5. Microglia but not astrocyte processes are anti-colocalized with Aβ42 hotspots
(a,b) Representative Confocal images of Aβ42 hotspot (arrowheads) and surrounding microglia. Microglia were labeled by cross breeding with _CX3CR1_-GFP mice. (b) Quantification of Aβ42-555 fluorescence in areas covered or uncovered by microglia. N=60 plaques from 3 mice. Data represents mean ± s.e.m. (unpaired t-test, t=(58)6.309). (c–e) Microglia coverage decreases as plaques grow while Aβ42 hotspot becomes more prominent. Small plaques tend to be completely wrapped by microglia without hotspot (d). Quantification of plaque perimeters in different size groups show that plaque size negatively correlated with microglia coverage, while positively correlated with Aβ42 hotspot (e). N=60 plaques from 3 mice. Data represents mean ± s.e.m; corresponding groups in each plaque size bin were compared with unpaired t-test (t=(30)2.728 and (22)2.218 for microglia coverage. t=(30)2.253 and (22)3.223 for Aβ42 coverage). (f,g) Astrocytic processes (labeled with GFAP) around amyloid plaques do not correlate with Aβ42 hotspot. N=60 plaques from 3 mice. Data represents mean ± s.e.m.. Scale bars: 5μm in all panels.
Figure 6. Unique plaque-labeling patterns of Aβ binding dyes
(a–h) Representative confocal images in fixed slices demonstrate unique labeling patterns for various β-sheet binding dyes. In vivo subarachnoid infusion of Aβ42-555 (red) was performed 1 day before slice dye labeling. Scale bars: 5μm. (i) Quantification of different β-sheet binding dyes labeling in Aβ42-555 hotspot and non-hotspot areas. N>10 plaques for each dye. Data represents mean ± s.e.m. (unpaired t-test, t=(14)3.210, (14)3.090 and (15)2.900).
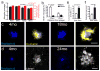
Figure 7. Microglia processes protect adjacent neurites from protofibrillar Aβ42 toxicity
(a–d) Representative confocal image analysis of the degree of microglia coverage (Iba1, green) versus extent of neuritic dystrophy (Lamp1, magenta or Ubiquitin, grey). Graphs in b, d represent the area of dystrophic neurites extending radially outward from microglia covered or uncovered plaque perimeter. The degree of neuritic dystrophy (Y axis) is normalized by the angular plaque coverage by microglia processes (see methods for detail). N>60 plaques from 3 mice. Data represents mean ± s.e.m. (paired t-test, t=(57)4.694 in (a) and (67)4.410 for (b)). (e,f) Representative confocal images of microglia and dystrophic neurites around amyloid plaques in Thy1-YFP/5xFAD mice. Quantification of dystrophic neurite number was done as in b and d. N=70 plaques from 4 mice. Data represents mean ± s.e.m. (paired t-test, t=(67)8.290). (g,h) Two-photon transcranial in vivo time-lapse images of dystrophic neurite dynamics around amyloid plaques. Newly formed (white arrowhead) and stable (yellow arrowhead) dystrophic neurites (labeled in either Thy1-YFP mice or by AAV-TdTomato injections) were quantified (data is a combination of both labeling methods). Data comes from 8 mice and 45 plaques. Data represents mean ± s.e.m. (paired t-test, t=(42)4.108). Scale bars represent 5μm in all panels.
Figure 8. Reduced microglia coverage in aging is associated with an increased Aβ halo and neurotoxicity
(a) Quantification of microglia coverage (black bars) and microglia number (red bars) within 25μm from the plaque edge at different ages in CRND8 mice. N>120 plaques from 4 mice per age group. Data represents mean ± s.e.m. (unpaired t-test, t=(373)3.905 and (286)5.602 in microglia coverage and t=(286)3.325 in microglia number). (b) Quantification of BrdU+ microglia in 4mo and 24mo CRND8 mice. N=222 and 100 field of views analyzed for 24 and 4 month-old mice, respectively. Data represents mean ± s.e.m. (unpaired t-test, t=(159)4.374). (c, d) Representative confocal images of Aβ halo (labeled with curcumin in c) and neuritic dystrophy (labeled with Lamp1 immunohistochemistry in d) in CRND8 mice. Scale bars represent 5μm in all panels. (e, f) Quantification of curcumin labeled protofibrillar Aβ halo (e) and Lamp1-labeled neuritic dystrophy area (f). N=62 plaques in (e) and 44 plaques in (f). Data represents mean ± s.e.m. (unpaired t-test, t=(60)5.508 for (e) and (40)2.404 for (f)).
Figure 9. CX3CR1 deletion or Aβ immunization increases microglia coverage and reduces dystrophy
(a) Representative confocal images of microglia coverage and dystrophic neurites in 8mo CRND8 mice harboring different copy numbers of CX3CR1 gene. (b) Quantification of microglia coverage as a function of CX3CR1 genotype and plaque size. t1=(140)7.465, t2=(159)8.939, t3=(202)5.890, t4=(249)13.28, t5=(56)3.760, t6=(47)5.784. (c) Quantification of dystrophic neurite area as a function of CX3CR1 genotype and plaque size. t7=(170)3.828, t8=(256)2.047. (d) Representative confocal images of microglia coverage and dystrophic neurites in 4mo 5xFAD mice treated with passive anti Aβ immunization for 8 weeks. (e) Quantification of microglia coverage as a function of treatment and plaque size. t1=(324)8.093, t2=(385)15.59, t3=(66)5.848. (f) Quantification of dystrophic neurite area as a function of treatment and plaque size. t4=(218)4.557. For all quantifications data represents mean ± s.e.m and compared using unpaired t-tests. In each genotype or treatment N>3 animals and 40 plaques per animal. Scale bars: 5μm in all panels.
Similar articles
- Trem2 Deletion Reduces Late-Stage Amyloid Plaque Accumulation, Elevates the Aβ42:Aβ40 Ratio, and Exacerbates Axonal Dystrophy and Dendritic Spine Loss in the PS2APP Alzheimer's Mouse Model.
Meilandt WJ, Ngu H, Gogineni A, Lalehzadeh G, Lee SH, Srinivasan K, Imperio J, Wu T, Weber M, Kruse AJ, Stark KL, Chan P, Kwong M, Modrusan Z, Friedman BA, Elstrott J, Foreman O, Easton A, Sheng M, Hansen DV. Meilandt WJ, et al. J Neurosci. 2020 Feb 26;40(9):1956-1974. doi: 10.1523/JNEUROSCI.1871-19.2019. Epub 2020 Jan 24. J Neurosci. 2020. PMID: 31980586 Free PMC article. - CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis.
Liu Z, Condello C, Schain A, Harb R, Grutzendler J. Liu Z, et al. J Neurosci. 2010 Dec 15;30(50):17091-101. doi: 10.1523/JNEUROSCI.4403-10.2010. J Neurosci. 2010. PMID: 21159979 Free PMC article. - Identification of highest neurotoxic amyloid-β plaque type showing reduced contact with astrocytes.
Mitsubori M, Takeda K, Nagashima S, Ishido S, Matsuoka M, Inatome R, Yanagi S. Mitsubori M, et al. Biochem Biophys Res Commun. 2021 Apr 16;549:67-74. doi: 10.1016/j.bbrc.2021.02.081. Epub 2021 Mar 2. Biochem Biophys Res Commun. 2021. PMID: 33667711 - Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease.
Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Nagele RG, et al. Neurobiol Aging. 2004 May-Jun;25(5):663-74. doi: 10.1016/j.neurobiolaging.2004.01.007. Neurobiol Aging. 2004. PMID: 15172746 Review. - Potential neurotoxic activity of diverse molecules released by microglia.
Lindhout IA, Murray TE, Richards CM, Klegeris A. Lindhout IA, et al. Neurochem Int. 2021 Sep;148:105117. doi: 10.1016/j.neuint.2021.105117. Epub 2021 Jun 27. Neurochem Int. 2021. PMID: 34186114 Review.
Cited by
- The deficient CLEC5A ameliorates the behavioral and pathological deficits via the microglial Aβ clearance in Alzheimer's disease mouse model.
Lin YY, Chang WH, Hsieh SL, Cheng IH. Lin YY, et al. J Neuroinflammation. 2024 Oct 23;21(1):273. doi: 10.1186/s12974-024-03253-x. J Neuroinflammation. 2024. PMID: 39443966 Free PMC article. - The antibody aducanumab reduces Aβ plaques in Alzheimer's disease.
Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O'Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. Sevigny J, et al. Nature. 2016 Sep 1;537(7618):50-6. doi: 10.1038/nature19323. Nature. 2016. PMID: 27582220 Updated. - Emerging Roles of Astrocytes in Neuro-Vascular Unit and the Tripartite Synapse With Emphasis on Reactive Gliosis in the Context of Alzheimer's Disease.
Liu CY, Yang Y, Ju WN, Wang X, Zhang HL. Liu CY, et al. Front Cell Neurosci. 2018 Jul 10;12:193. doi: 10.3389/fncel.2018.00193. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30042661 Free PMC article. Review. - Bidirectional Microglia-Neuron Communication in Health and Disease.
Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Szepesi Z, et al. Front Cell Neurosci. 2018 Sep 27;12:323. doi: 10.3389/fncel.2018.00323. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30319362 Free PMC article. Review. - Pathological changes induced by Alzheimer's brain inoculation in amyloid-beta plaque-bearing mice.
Lam S, Hérard AS, Boluda S, Petit F, Eddarkaoui S, Cambon K; Brainbank Neuro-CEB Neuropathology Network; Picq JL, Buée L, Duyckaerts C, Haïk S, Dhenain M. Lam S, et al. Acta Neuropathol Commun. 2022 Aug 16;10(1):112. doi: 10.1186/s40478-022-01410-y. Acta Neuropathol Commun. 2022. PMID: 35974399 Free PMC article.
References
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–81. - PubMed
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AG048181/AG/NIA NIH HHS/United States
- P50 AG047270/AG/NIA NIH HHS/United States
- R21 NS088411/NS/NINDS NIH HHS/United States
- S10 RR024746/RR/NCRR NIH HHS/United States
- R01AG027855/AG/NIA NIH HHS/United States
- R01 AG027855/AG/NIA NIH HHS/United States
- R01 NS089734/NS/NINDS NIH HHS/United States
- R01HL106815/HL/NHLBI NIH HHS/United States
- R01 NS089662/NS/NINDS NIH HHS/United States
- R01 HL106815/HL/NHLBI NIH HHS/United States
- R21 NS087511/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous