Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus - PubMed (original) (raw)
doi: 10.1038/cmi.2015.03. Epub 2015 Feb 2.
Rudragouda Channappanavar 2, Cuiqing Ma 1, Lili Wang 1, Jian Tang 1 3 4, Tania Garron 5, Xinrong Tao 5, Sumaiya Tasneem 1, Lu Lu 6, Chien-Te K Tseng 5, Yusen Zhou 3 4, Stanley Perlman 2, Shibo Jiang 1 6, Lanying Du 1
Affiliations
- PMID: 25640653
- PMCID: PMC4786625
- DOI: 10.1038/cmi.2015.03
Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus
Naru Zhang et al. Cell Mol Immunol. 2016 Mar.
Abstract
Middle East respiratory syndrome (MERS), an emerging infectious disease caused by MERS coronavirus (MERS-CoV), has garnered worldwide attention as a consequence of its continuous spread and pandemic potential, making the development of effective vaccines a high priority. We previously demonstrated that residues 377-588 of MERS-CoV spike (S) protein receptor-binding domain (RBD) is a very promising MERS subunit vaccine candidate, capable of inducing potent neutralization antibody responses. In this study, we sought to identify an adjuvant that optimally enhanced the immunogenicity of S377-588 protein fused with Fc of human IgG (S377-588-Fc). Specifically, we compared several commercially available adjuvants, including Freund's adjuvant, aluminum, Monophosphoryl lipid A, Montanide ISA51 and MF59 with regard to their capacity to enhance the immunogenicity of this subunit vaccine. In the absence of adjuvant, S377-588-Fc alone induced readily detectable neutralizing antibody and T-cell responses in immunized mice. However, incorporating an adjuvant improved its immunogenicity. Particularly, among the aforementioned adjuvants evaluated, MF59 is the most potent as judged by its superior ability to induce the highest titers of IgG, IgG1 and IgG2a subtypes, and neutralizing antibodies. The addition of MF59 significantly augmented the immunogenicity of S377-588-Fc to induce strong IgG and neutralizing antibody responses as well as protection against MERS-CoV infection in mice, suggesting that MF59 is an optimal adjuvant for MERS-CoV RBD-based subunit vaccines.
Figures
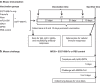
Figure 1
Schematic diagram of immunization and challenge strategies and sample collection. (a) Mouse immunization. Groups of mice (five mice/group) were subcutaneously immunized with MERS-CoV S377–588-F_c_ protein alone or with a selected adjuvant, as indicated, followed by two boosters at 21 days apart from each immunization. Sera were collected at 0 and 10 days post-each immunization and tested for titers of MERS-CoV S1-specific IgG, IgG1 and IgG2a antibodies, and neutralizing antibodies. Splenocytes were also collected at day 10 after the last immunization and processed for evaluating MERS-CoV S1-specific T cell-mediated responses, as described in the section on ‘Materials and methods'. (b) Mouse challenge. Mice immunized with S377–588-F_c_ or PBS control plus MF59 adjuvant were transduced with Ad5-hDPP4 at day 10 after the last immunization, and challenged with MERS-CoV 5 days later. Lung tissues were harvested at days 3 and 5, respectively, post-challenge to detect virus titers. MERS, Middle East respiratory syndrome; MERS-CoV, MERS coronavirus.
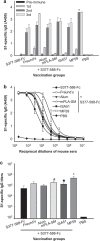
Figure 2
Formulation of MERS-CoV S377–588-F_c_ protein with different adjuvants enhanced MERS-CoV S1-specific IgG antibody responses at varying extents elicited by immunized mice. Sera collected at different days after each immunization, as depicted in Figure 1, were subjected to ELISA-based analysis for S1-specific IgG antibody responses. IgG antibody titers were evaluated by using serum specimens that were diluted 3200-fold. Sera collected from mice prior to immunization, i.e., day 0 and day 10 after each immunization were included in this evaluation for determining the kinetics of IgG antibody response (a). Additionally, sera collected from individual groups of mice at day 10 after the last immunization were also subjected to the ELISA-based binding (b) and specific IgG antibody assays (c), respectively. The titers are expressed as the endpoint dilutions that remain positively detectable. The data are presented as mean±s.d. from five mice in each group. PBS was included as the control. Significant differences in the responses were observed between MF59 and other groups (*), between mPLA-SM and Alum or S377–588-F_c_ (#) or between ISA51 and S377–588-F_c_ (▪). MERS, Middle East respiratory syndrome; MERS-CoV, MERS coronavirus.
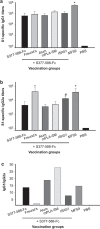
Figure 3
Titers of serum IgG1 and IgG2a antibodies to MERS-CoV S1 protein elicited by MERS-CoV S377–588-F_c_ protein-immunized mice with or without adjuvants. Sera collected at day 10 after the last immunization were used to determine the nature of Th1-versus_-Th2 IgG antibody responses by quantifying MERS-CoV S1-specific IgG1 (Th2) (a) and IgG2a (Th1) (b), respectively. In addition, the ratios between specific IgG1 and IgG2a antibody responses were calculated and expressed for assessing the nature of antibody responses (c). The antibody titers were expressed as the endpoint dilutions that remain positively detectable. The data are presented as mean±s.d. from five mice in each group. PBS was included as the control. (a) Significant differences in the IgG1 responses were observed between MF59 and other groups (*). (b) Significant differences in the IgG2a responses were observed in groups immunized with MF59- or Freund's-adjuvanted S377–588-F_c, compared to other vaccinated groups (*). Such a significant difference was also observed between ISA51- and Alum-adjuvanted groups (#). MERS, Middle East respiratory syndrome; MERS-CoV, MERS coronavirus.
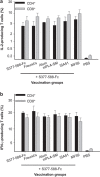
Figure 4
Mice vaccinated with MERS-CoV S377–588-F_c_ protein in the absence or presence of adjuvants also resulted in the induction of antigen-specific T cell-mediated responses. The capacity of vaccination-induced MERS-CoVS1-specific T cell-mediated responses in mice were assessed by using the standard FACS-based analysis to quantify the frequencies of IL-2- and IFN-γ-producing CD4+ and CD8+ T cells, as described in the section on ‘Materials and methods'. Briefly, splenocytes extracted from mice at day 10 after the last immunization were subjected to incubation with recombinant MERS-CoV S1 protein and the frequencies of IL-2 (a) and IFN-γ (b) producing cells are expressed as percentages of CD4+ or CD8+ T cells. The samples were tested in triplicate and presented as means±s.d. from five mice in each group. MERS, Middle East respiratory syndrome; MERS-CoV, MERS coronavirus.
Figure 5
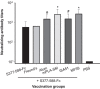
Mice immunized with different MERS-CoV S377–588-F_c_ protein-based vaccine formulations were capable of promoting neutralizing antibody responses to varying extents. Sera obtained from mice at day 10 after the last immunization were evaluated for their content of neutralization antibody against live MERS-CoV, based on Vero E6 cell-based microneutralization assays. Neutralizing antibody titers are expressed as the reciprocal of the highest dilutions of specimens that resulted in a complete inhibition of virus-induced CPE in at least 50% of the wells (NT50), and the data are presented as mean±s.d. from five mice in each group. There were significant differences between two adjuvants (MF59, mPLA-SM) and other groups (*) or between two adjuvants (Alum, ISA51) and Freund's or S377–588-F_c_ group (#). CPE, cytopathic effect; MERS, Middle East respiratory syndrome; MERS-CoV, MERS coronavirus.
Figure 6
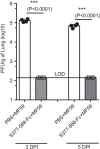
The in vivo efficacy of MERS-CoV S377–588-F_c_ protein-based vaccine formulated with MF59 adjuvant in protection of Ad5-hDPP4-transduced mice challenged with MERS-CoV. BALB/c mice were immunized with S377–588-F_c_ plus MF59 adjuvant. PBS plus MF59 was included as the control. Ten days post-last immunization, mice were transduced with Ad5-hDPP4, and then i.n. infected with MERS-CoV as described in the section on ‘Materials and methods'. Three (3 DPI) and five (5 DPI) days post-infection, virus titers were determined in lung tissues, and expressed as PFU/g of lung tissues. *** indicates significant differences between the two groups (P<0.0001). i.n., intranasally; LOD, limit of detection; MERS, Middle East respiratory syndrome; MERS-CoV, MERS coronavirus; PFU, plaque forming units.
Similar articles
- Antibodies and vaccines against Middle East respiratory syndrome coronavirus.
Xu J, Jia W, Wang P, Zhang S, Shi X, Wang X, Zhang L. Xu J, et al. Emerg Microbes Infect. 2019;8(1):841-856. doi: 10.1080/22221751.2019.1624482. Emerg Microbes Infect. 2019. PMID: 31169078 Free PMC article. Review. - Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus.
Tang J, Zhang N, Tao X, Zhao G, Guo Y, Tseng CT, Jiang S, Du L, Zhou Y. Tang J, et al. Hum Vaccin Immunother. 2015;11(5):1244-50. doi: 10.1080/21645515.2015.1021527. Hum Vaccin Immunother. 2015. PMID: 25874632 Free PMC article. - Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments--the importance of immunofocusing in subunit vaccine design.
Ma C, Wang L, Tao X, Zhang N, Yang Y, Tseng CK, Li F, Zhou Y, Jiang S, Du L. Ma C, et al. Vaccine. 2014 Oct 21;32(46):6170-6176. doi: 10.1016/j.vaccine.2014.08.086. Epub 2014 Sep 19. Vaccine. 2014. PMID: 25240756 Free PMC article. - Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants.
Tai W, Wang Y, Fett CA, Zhao G, Li F, Perlman S, Jiang S, Zhou Y, Du L. Tai W, et al. J Virol. 2016 Dec 16;91(1):e01651-16. doi: 10.1128/JVI.01651-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795425 Free PMC article. - Receptor-binding domain-based subunit vaccines against MERS-CoV.
Zhang N, Tang J, Lu L, Jiang S, Du L. Zhang N, et al. Virus Res. 2015 Apr 16;202:151-9. doi: 10.1016/j.virusres.2014.11.013. Epub 2014 Nov 20. Virus Res. 2015. PMID: 25445336 Free PMC article. Review.
Cited by
- Comparing the Immunogenicity and Protective Effects of Three MERS-CoV Inactivation Methods in Mice.
Kim N, Lee TY, Lee H, Yang JS, Kim KC, Lee JY, Kim HJ. Kim N, et al. Vaccines (Basel). 2022 Oct 31;10(11):1843. doi: 10.3390/vaccines10111843. Vaccines (Basel). 2022. PMID: 36366352 Free PMC article. - Prospect of SARS-CoV-2 spike protein: Potential role in vaccine and therapeutic development.
Samrat SK, Tharappel AM, Li Z, Li H. Samrat SK, et al. Virus Res. 2020 Oct 15;288:198141. doi: 10.1016/j.virusres.2020.198141. Epub 2020 Aug 23. Virus Res. 2020. PMID: 32846196 Free PMC article. Review. - A randomized phase I/II safety and immunogenicity study of the Montanide-adjuvanted SARS-CoV-2 spike protein-RBD-Fc vaccine, AKS-452.
Feitsma EA, Janssen YF, Boersma HH, van Sleen Y, van Baarle D, Alleva DG, Lancaster TM, Sathiyaseelan T, Murikipudi S, Delpero AR, Scully MM, Ragupathy R, Kotha S, Haworth JR, Shah NJ, Rao V, Nagre S, Ronca SE, Green FM, Aminetzah A, Sollie F, Kruijff S, Brom M, van Dam GM, Zion TC. Feitsma EA, et al. Vaccine. 2023 Mar 24;41(13):2184-2197. doi: 10.1016/j.vaccine.2023.02.057. Epub 2023 Feb 23. Vaccine. 2023. PMID: 36842886 Free PMC article. Clinical Trial. - Effects of Adjuvants on the Immunogenicity and Efficacy of a Zika Virus Envelope Domain III Subunit Vaccine.
Wang X, Tai W, Zhang X, Zhou Y, Du L, Shen C. Wang X, et al. Vaccines (Basel). 2019 Oct 27;7(4):161. doi: 10.3390/vaccines7040161. Vaccines (Basel). 2019. PMID: 31717890 Free PMC article. - Antibodies and vaccines against Middle East respiratory syndrome coronavirus.
Xu J, Jia W, Wang P, Zhang S, Shi X, Wang X, Zhang L. Xu J, et al. Emerg Microbes Infect. 2019;8(1):841-856. doi: 10.1080/22221751.2019.1624482. Emerg Microbes Infect. 2019. PMID: 31169078 Free PMC article. Review.
References
- Zaki AM, van BS, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367: 1814–1820. - PubMed
- Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 2014; 370: 2499–2505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01AI06099/AI/NIAID NIH HHS/United States
- R21 AI109094/AI/NIAID NIH HHS/United States
- R01 AI091322/AI/NIAID NIH HHS/United States
- R21AI109094/AI/NIAID NIH HHS/United States
- P01 AI060699/AI/NIAID NIH HHS/United States
- T32 AI060549/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources