Transient and sustained afterdepolarizations in accessory olfactory bulb mitral cells are mediated by distinct mechanisms that are differentially regulated by neuromodulators - PubMed (original) (raw)
Transient and sustained afterdepolarizations in accessory olfactory bulb mitral cells are mediated by distinct mechanisms that are differentially regulated by neuromodulators
Guy Shpak et al. Front Cell Neurosci. 2015.
Abstract
Social interactions between mammalian conspecifics rely heavily on molecular communication via the main and accessory olfactory systems. These two chemosensory systems show high similarity in the organization of information flow along their early stages: social chemical cues are detected by the sensory neurons of the main olfactory epithelium and the vomeronasal organ. These neurons then convey sensory information to the main (MOB) and accessory (AOB) olfactory bulbs, respectively, where they synapse upon mitral cells that project to higher brain areas. Yet, the functional difference between these two chemosensory systems remains unclear. We have previously shown that MOB and AOB mitral cells exhibit very distinct intrinsic biophysical properties leading to different types of information processing. Specifically, we found that unlike MOB mitral cells, AOB neurons display persistent firing responses to strong stimuli. These prolonged responses are mediated by long-lasting calcium-activated non-selective cationic current (Ican). In the current study we further examined the firing characteristics of these cells and their modulation by several neuromodulators. We found that AOB mitral cells display transient depolarizing afterpotentials (DAPs) following moderate firing. These DAPs are not found in MOB mitral cells that show instead robust hyperpolarizing afterpotentials. Unlike Ican, the DAPs of AOB mitral cells are activated by low levels of intracellular calcium and are relatively insensitive to flufenamic acid. Moreover, the cholinergic agonist carbachol exerts opposite effects on the persistent firing and DAPs of AOB mitral cells. We conclude that these phenomena are mediated by distinct biophysical mechanisms that may serve to mediate different types of information processing in the AOB at distinct brain states.
Keywords: accessory olfactory bulb; carbachol; depolarizing afterpotential; electrophysiological properties; hyperpolarizing afterpotential; neuromodulators; vomeronasal system.
Figures
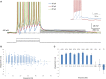
Figure 1
AOB mitral cells exhibit depolarizing afterpotential (DAP). (A) Representative superimposed voltage traces of firing responses (spikes cut at 0 mV) to three different depolarizing current steps (20, 50, and 80 pA) in the same cell (inter stimulus interval = 5 s). The DAP integral was measured by calculating the integral of the voltage traces relative the membrane baseline (dashed line), during the first second (gray bar) following the termination of the current step. Inset: Example traces from another cell showing marked ADP following low firing rate (blue) as opposed to HAP with high firing rate (red). (B) The DAP integral as a function of the firing frequency of 1309 stimuli given to 71 AOB mitral cells. Stimulation amplitude ranged between 5 and 150 pA. Max stimulation amplitude was determined by the saturation of the I-F response. (C) Mean (±s.e.m.) values of the data shown in (B), using 5 Hz bins (n of each frequency in brackets above).
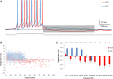
Figure 2
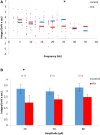
AOB and MOB mitral cells exhibit opposite afterpotentials in response to depolarizing current injections. (A) Superimposed MOB (red) and AOB (blue) voltage responses to a 30 pA square-pulse depolarizing current injection. Spikes were cut at 0 mV. (B) The calculated DAP integral as a function of the firing frequency in 1317 stimuli given to 70 AOB cells, and 254 stimuli given to 14 MOB cells. (C) Mean (±s.e.m.) values of the data shown in (B), using 10 Hz bins.
Figure 3
The DAPs of AOB mitral cells depend on low levels of intracellular calcium. (A) DAP integral of AOB mitral cells plotted as a function of firing frequency during stimulation in control (blue, n = 43 cells, 1345 stimuli) and 5 mM BAPTA-filled cells (green, n = 11 cells, 256 stimuli). Horizontal colored lined represent the mean using 5 Hz bins. (B) Mean (±s.e.m.) values of the responses to three current amplitudes representing low (20 pA), moderate (50 pA), and high (80 pA) stimulation levels. A significant difference (*p < 0.05, _t_-test corrected for multiple comparisons) was found between control and BAPTA conditions only for low and moderate stimulation levels.
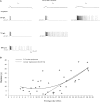
Figure 4
Sustained firing responses of AOB mitral cells require intensive firing episode. (A) Responses of a single AOB mitral cell to increasing amplitude (rows) or decay time constant (columns) of EPSC-like current injection. (B) Response duration plotted as a function of the mean firing rate at the first second of the response (n = 35 stimuli given to 8 cells of 6 animals). A 2nd-order polinom fitted the data, suggesting two types of relationships. Separating the data to two sets at firing rate of 13 Hz was found to yield the best fit of two linear regressions. Accordingly, a statistically significant correlation is observed for firing rates of ≥13 Hz (_R_2 = 0.6, p < 0.001, Spearman's) while no correlation is observed for mean firing rates of <13 Hz (_R_2 = 0.16, _p_ > 0.2).
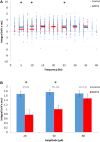
Figure 5
The DAPs of AOB mitral cells are only partially sensitive to flufenamic acid (FFA). (A) DAP integral of AOB mitral cells plotted as a function of firing frequency evoked by square pulse stimulation under control conditions (blue, n = 5 cells, 79 stimuli) and in the presence of 50 μM FFA (green, same 5 cells, 80 stimuli). (B) Mean (±s.e.m.) values of the responses to three current amplitudes representing low (20 pA), moderate (50 pA), and high (80 pA) stimulation levels. A significant difference (*p < 0.05, paired _t_-test corrected for multiple comparisons) was found between control and BAPTA conditions only for low level of stimulation.
Figure 6
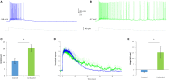
Carbachol enhances the sustained firing response in AOB mitral cells. (A,B) Typical voltage responses of an AOB mitral cell to injection of EPSC-like current (40 pA, gray trace below) in the absence (A) and presence (B) of Carbachol (1 μM) in the bath solution. The injected current amplitude was selected to be below the level sufficient for persistent firing induction in this cell under control condition (weak current injection). (C) Comparison of the firing duration of five cells (from four animals) to 20 pA EPSC-like current injection in control and carbachol conditions (*p < 0.05, paired _t_-test). (D) Mean normalized voltage trajectories of the responses of the same cells as in (C), recorded before (blue) and after (green) carbachol application to the bath solution. Dashed lines represent SD values to one direction. (E) Comparison of the integral (20–30 s post-stimulation) under the voltage trajectories of the same cells (without normalization) between control and carbachol conditions (*p < 0.05, paired _t_-test).
Figure 7
Carbachol blocks DAPs in AOB mitral cells. (A) Mean current-frequency (I-F) curves of the same cells as in Figure 6 before (blue) and after (green) carbachol (1 μM) application to the bath solution. (B) Representative voltage responses to 40 pA square-pulse stimulation of the same cell in control and carbachol conditions. Spikes were cut at 0 mV. (C) Mean (±s.e.m.) DAP integral of all responses of the same cells, plotted as a function of the firing frequencies. Horizontal lines represent the mean using 10 Hz bins. (D) Mean (±s.e.m.) values of the DAP integral of the responses to three current amplitudes representing low (20 pA), moderate (50 pA), and high (80 pA) stimulation levels. A significant difference (*p < 0.05, paired _t_-test corrected for multiple comparisons) was found between control and carbachol conditions for all stimulation levels.
Similar articles
- Differential Muscarinic Modulation in the Olfactory Bulb.
Smith RS, Hu R, DeSouza A, Eberly CL, Krahe K, Chan W, Araneda RC. Smith RS, et al. J Neurosci. 2015 Jul 29;35(30):10773-85. doi: 10.1523/JNEUROSCI.0099-15.2015. J Neurosci. 2015. PMID: 26224860 Free PMC article. - Calcium-activated sustained firing responses distinguish accessory from main olfactory bulb mitral cells.
Shpak G, Zylbertal A, Yarom Y, Wagner S. Shpak G, et al. J Neurosci. 2012 May 2;32(18):6251-62. doi: 10.1523/JNEUROSCI.4397-11.2012. J Neurosci. 2012. PMID: 22553031 Free PMC article. - Differential serotonergic modulation across the main and accessory olfactory bulbs.
Huang Z, Thiebaud N, Fadool DA. Huang Z, et al. J Physiol. 2017 Jun 1;595(11):3515-3533. doi: 10.1113/JP273945. Epub 2017 Mar 31. J Physiol. 2017. PMID: 28229459 Free PMC article. - Vomeronasal Receptors and Signal Transduction in the Vomeronasal Organ of Mammals.
Francia S, Pifferi S, Menini A, Tirindelli R. Francia S, et al. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 10. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 10. PMID: 24830038 Free Books & Documents. Review. - Histological properties of the glomerular layer in the mouse accessory olfactory bulb.
Yokosuka M. Yokosuka M. Exp Anim. 2012;61(1):13-24. doi: 10.1538/expanim.61.13. Exp Anim. 2012. PMID: 22293668 Review.
Cited by
- Electrophysiological Evidence for a Direct Link between the Main and Accessory Olfactory Bulbs in the Adult Rat.
Vargas-Barroso V, Ordaz-Sánchez B, Peña-Ortega F, Larriva-Sahd JA. Vargas-Barroso V, et al. Front Neurosci. 2016 Jan 26;9:518. doi: 10.3389/fnins.2015.00518. eCollection 2015. Front Neurosci. 2016. PMID: 26858596 Free PMC article. - Differential Muscarinic Modulation in the Olfactory Bulb.
Smith RS, Hu R, DeSouza A, Eberly CL, Krahe K, Chan W, Araneda RC. Smith RS, et al. J Neurosci. 2015 Jul 29;35(30):10773-85. doi: 10.1523/JNEUROSCI.0099-15.2015. J Neurosci. 2015. PMID: 26224860 Free PMC article. - Neural mechanisms of social learning in the female mouse.
Gao Y, Budlong C, Durlacher E, Davison IG. Gao Y, et al. Elife. 2017 Jun 16;6:e25421. doi: 10.7554/eLife.25421. Elife. 2017. PMID: 28621665 Free PMC article. - Prolonged Intracellular Na+ Dynamics Govern Electrical Activity in Accessory Olfactory Bulb Mitral Cells.
Zylbertal A, Kahan A, Ben-Shaul Y, Yarom Y, Wagner S. Zylbertal A, et al. PLoS Biol. 2015 Dec 16;13(12):e1002319. doi: 10.1371/journal.pbio.1002319. eCollection 2015 Dec. PLoS Biol. 2015. PMID: 26674618 Free PMC article.
References
- Aroniadou-Anderjaska V., Ennis M., Shipley M. T. (1999). Dendrodendritic recurrent excitation in mitral cells of the rat olfactory bulb. J. Neurophysiol. 82, 489–494. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources