Structural basis for assembly and function of the Nup82 complex in the nuclear pore scaffold - PubMed (original) (raw)
. 2015 Feb 2;208(3):283-97.
doi: 10.1083/jcb.201411003.
Dirk Flemming 1, Alexander von Appen 2, Panagiotis Kastritis 2, Norbert Mücke 3, Jessica Fischer 1, Philipp Stelter 1, Alessandro Ori 2, Khanh Huy Bui 2, Jochen Baßler 1, Elisar Barbar 4, Martin Beck 5, Ed Hurt 6
Affiliations
- PMID: 25646085
- PMCID: PMC4315244
- DOI: 10.1083/jcb.201411003
Structural basis for assembly and function of the Nup82 complex in the nuclear pore scaffold
Monika Gaik et al. J Cell Biol. 2015.
Abstract
Nuclear pore complexes (NPCs) are huge assemblies formed from ∼30 different nucleoporins, typically organized in subcomplexes. One module, the conserved Nup82 complex at the cytoplasmic face of NPCs, is crucial to terminate mRNA export. To gain insight into the structure, assembly, and function of the cytoplasmic pore filaments, we reconstituted in yeast the Nup82-Nup159-Nsp1-Dyn2 complex, which was suitable for biochemical, biophysical, and electron microscopy analyses. Our integrative approach revealed that the yeast Nup82 complex forms an unusual asymmetric structure with a dimeric array of subunits. Based on all these data, we developed a three-dimensional structural model of the Nup82 complex that depicts how this module might be anchored to the NPC scaffold and concomitantly can interact with the soluble nucleocytoplasmic transport machinery.
© 2015 Gaik et al.
Figures
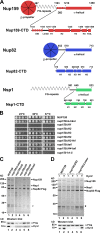
Figure 1.
The Nup159 CTD is important for Nup82 complex assembly and incorporation into the NPC scaffold. (A) Domain architecture of yeast Nup159, Nup82, and Nsp1. Amino acid positions are indicated above, and sequence motifs are given below the drawings. α-Helically predicted C-terminal domains (CTD) are shown in enlarged schemes (not in scale) according to secondary structure predictions. (B) Growth analysis of wild-type and nup159 deletion strains. A nup159Δ shuffle strain was transformed with the indicated nup159 mutant constructs under the control of the endogenous NUP159 promoter. It was spotted in 10-fold serial dilutions onto synthetic dextrose complete medium + 5-FOA plates and incubated at the indicated temperatures for 5 d. (C and D) Mutations in the Nup159 CTD impair in vivo Nup82 complex assembly and Dyn2 recruitment. Tandem affinity-purified Nup82-Flag-TEV-ProtA eluates derived from cells expressing wild-type or the indicated Nup159-CTD mutant constructs were analyzed by SDS-PAGE and Coomassie staining (top) or Western blotting using anti-Flag (to detect Nup82) and anti-Dyn2 antibodies (bottom). Indicated on the right are the Nup159, Nsp1, and Nup82-Flag bands; #, TEV protease. White lines indicate that intervening lanes have been spliced out. (D) The nup159ΔLinker mutant can only bind to Dyn2, when overexpressed. See also
Fig. S1, Fig. S2 A, and Tables S1 and S2.
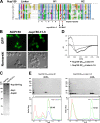
Figure 2.
Heptad repeats in the H1 subdomain of Nup159-CTD are crucial for self-dimerization and Nup82 complex assembly. (A) Multisequence alignment of Nup159 CTDs from the indicated species: C.t, Chaetomium thermophilum; N.c., Neurospora crassa; S.m., Sordaria macrospora; P.a., Podospora anserina; L.t., Lachancea thermotolerans; C.g., Candida glabrata; P.s., Pichia stipitis; Z.r., Zygosaccharomyces rouxii; V.p., Vanderwaltozyma polyspora; A.g., Ashbya gossypii; S.c., Saccharomyces cerevisiae. The Nup159 H1 subdomain has conserved heptad repeats with hydrophobic residues at position 1 and 4 (indicated below). Arrows point to the amino acid mutations in the nup159 h1-5 mutant (Ile1232>Asp/Met1235>Glu). The default color scheme of ClustalX/Jalview was used with e.g., hydrophobic residues in blue, acidic residues in violet, and basic residues in red. (B) Subcellular location of wild-type Nup159 and mutant Nup159 h1-5, both labeled with GFP, in yeast cells grown at 30°C. Mutant nup159 h1-5 has lost the characteristic nuclear rim (NPC) staining. Bars, 2 µm. (C) nup159 h1-5 is defective in Nup82 complex assembly. Affinity-purified Nup159-Flag-TEV-ProtA eluates derived from cells grown at 30°C and expressing either wild-type or mutant nup159 h1-5 were analyzed by SDS-PAGE and Coomassie staining. Nup159 h1-5-Flag-TEV-ProtA was not coenriched for Nup82 and Nsp1. (D) Far UV CD spectra of E. coli expressed and affinity-purified Nup159-QT4–5-Linker-H1 and Nup159-QT4–5-Linker-h1-5 mutant constructs. Nup159-H1 exhibits an overall α-helical structure indicated by two minima at 208 and 222 nm. In contrast, the Nup159-h1-5 mutant construct is fully disordered as shown by a single signal at 203 nm and absence of a signal at 222 nm. (E) Recombinant Nup159-H1 subdomain forms a homo-oligomer, which is disassembled upon mutating the heptad repeat pattern. The indicated Nup159-QT4–5-Linker-H1 wild-type and mutant fragments (see also D) were affinity purified and separated by SEC followed by SDS-PAGE and Coomassie staining (top) or analyzed by MALS (bottom). Intact Nup159-QT4–5-Linker-H1 construct eluted as a homotetramer (52 kD), whereas Nup159-QT4–5-Linker_-h1-5_ mutant construct was monomeric (13 kD) but also found in large aggregates. The data shown are from a single representative experiment out of two repeats. See also
Fig. S1
. dRI, differential refractive index; L, load; LS, light scattering; MM, molecular mass.
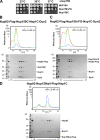
Figure 3.
SEC-MALS analysis of the different in vivo assembled Nup82 complexes. (A) Growth analysis of yeast _nup159Δ_-null strain complemented with the indicated Nup159 constructs under control of the GAL1 promoter and used for biochemical and EM analyses. Specifically, the nup159Δ shuffle strain was transformed with LEU2 plasmids carrying wild-type NUP159, nup159ΔFG, and nup159C, respectively. Subsequently, the _URA3_-NUP159 shuffle plasmid was shuffled out on galactose-containing 5-FOA plates. Derived yeast colonies complemented by NUP159, nup159ΔFG, and nup159C were spotted onto YPG (yeast extract, peptone, galactose) plates before it was further grown for 5 d. (B) SEC-MALS analysis of the affinity-purified Nup82–Nup159C–Nsp1C–Dyn2 complex, assembled in yeast cells as described in Materials and methods. The differential refractive index (dRI), light scattering (LS), and UV graphs are plotted against the elution volumes from a Superdex 200 Increase 10/300 GL gel filtration column. Two individual Nup82 complex preparations were analyzed by SEC-MALS, yielding a rather high molecular mass (MM) in the range of 650 kD (645 and 672 kD, respectively). The data shown are from a single representative experiment out of two repeats. The white line on the Coomassie-stained gel indicates that the intervening lanes corresponding to fractions 12–14 were removed for presentation purposes. (C) SEC-MALS analysis of the affinity-purified Nup82–Nup159ΔFG–Nsp1C–Dyn2 complex, revealing a molecular mass of 744 kD (calculated molecular mass of Nup159ΔFG, 100 kD). The data shown are from a single representative experiment out of two repeats. (D) SEC-MALS analysis of the affinity-purified Nup82–Nup159tail–Flag-Nsp1C complex, revealing a molecular mass of 114 kD (Nup159 tail fragment has a calculated molecular mass of 8 kD). The data shown are from a single representative experiment out of two repeats. See also
Fig. S2 (B–E)
. L, load.
Figure 4.
EM analysis of the purified Nup82 complexes. (A and B) Electron micrographs of the affinity-purified and GraFix-treated Nup82-Flag–Nup159C–Nsp1C–Dyn2 complex (A) or Nup82-Flag–Nup159ΔFG–Nsp1C–Dyn2 complex (B). Bars, 50 nm. Shown are an overview picture (left), a gallery of the representative class averages determined by multivariate statistical analysis (middle), and two enlarged classes, displaying presumable front and back orientation of the Nup82 complex (right). Arrows indicate distinct globular masses, corresponding to Nup82 β-propeller domains (A) and Nup159 β-propeller domains (B). See also
Fig. S3
.
Figure 5.
3D structural analysis of the Nup82–Nup159C–Nsp1C–Dyn2 complex by negative-staining electron tomography. (A) Representative class averages of affinity-purified Nup82–Nup159C–Nsp1C–Dyn2 complex based on subtomogram averaging. Parts of the DIDNup159-Dyn2 stalk are averaged out in the first two classes. (B) Representative class averages of the affinity-purified Nup82–Nup159C–Nsp1C–Dyn2 complex based on subtomogram averaging focused by local masking to the head region such that it is resolved with higher detail. (C) Representative class of the affinity-purified Nup82–Nup159C–Nsp1C–Dyn2 complex. Three globular domains and a spurlike element orthogonal to the stalk build the backbone structure. The globular domains 3 and 1 are connected by a rod element. See also
Fig. S4 (A and B)
.
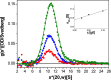
Figure 6.
Sedimentation velocity analysis of the Nup82 complex. Sedimentation coefficient distribution of the Nup82–Nup159C–Nsp1C–Dyn2 complex measured at three protein concentrations (c), 100 µg/ml (green squares), 50 µg/ml (blue squares), and 25 µg/ml (red squares). Gaussian functions (lines) were fitted to the data. Peak values were extrapolated to a protein concentration of 0 µg/ml (inset), and an s0,20,w value for the main fraction of 10.4 S was observed. The slight increase of the s value with increasing concentration indicates a reversible interaction of the Nup82–Nup159C–Nsp1C–Dyn2 complex. The data shown are from a single representative experiment out of two repeats.
Figure 7.
Quantitative MS, XL-MS, and structural modeling of the Nup82–Nup159C–Nsp1C–Dyn2 complex. (A) XL-MS of the affinity-purified Nup82–Nup159C–Nsp1C–Dyn2 complex using DSS. The primary structure of the protein is shown, and specific regions are indicated. Interprotein cross-links are shown in orange; intraprotein cross-links are in purple. For the visualization of cross-links, the xiNET tool from the Rappsilber laboratory was used (
http://crosslinkviewer.org/index.php
). Because multiple copies of each protein are present, the latter might also occur across multiple instances of the same protein. Homodimeric cross-links that connect the two instances of the same lysine residue are indicated with red arrows. (B) Stoichiometry measurements of purified Nup82–Nup159C–Nsp1C–Dyn2 complex by quantitative, targeted proteomics within the apex of the gel filtration peak (Fig. 3 B). Two heavy-labeled reference peptides per protein were used as intrinsic standards. The apparent values were normalized to the abundance of Nup159; error bars correspond to one standard deviation. (C) Structural model illustrating the possible architecture of the Nup82–Nup159C–Nsp1C–Dyn2 complex. Two Nup82–Nup159 heterodimers are shown in green and blue, as well as green and orange, and occupy globular domains 1 and 3, respectively. The N terminus of the Nup159 might proceed through the loop toward Dyn2-DIDNup159 stalk (only two of five Dyn2 dimers are shown). The Nup159 H1 domains (gray helices) reside closely to the Dyn2-DIDNup159 stalk as shown by the XL-MS analysis. See also
Figs. S4 and S5 and Tables S3–S5
.
Figure 8.
Potential position of the Nup82–Nup159C–Nsp1C–Dyn2 complex within the human NPC. (A) Three orthogonal slices through the cytosolic part (top), the central channel (middle), and the nucleoplasmic part (bottom) of the previously published tomographic map of the human NPC are shown. Red circles indicate a region in which additional density is observed in the cytoplasmic ring that could accommodate the P complex. (B) Two possible placements (middle and bottom) of the Nup82–Nup159C–Nsp1C–Dyn2 complex (blue) within the tomographic map of the NPC (top). The progression of the Dyn2-DIDNup159 stalk is indicated by dashed arrows.
Similar articles
- Structure of a yeast Dyn2-Nup159 complex and molecular basis for dynein light chain-nuclear pore interaction.
Romes EM, Tripathy A, Slep KC. Romes EM, et al. J Biol Chem. 2012 May 4;287(19):15862-73. doi: 10.1074/jbc.M111.336172. Epub 2012 Mar 12. J Biol Chem. 2012. PMID: 22411995 Free PMC article. - Molecular basis for the functional interaction of dynein light chain with the nuclear-pore complex.
Stelter P, Kunze R, Flemming D, Höpfner D, Diepholz M, Philippsen P, Böttcher B, Hurt E. Stelter P, et al. Nat Cell Biol. 2007 Jul;9(7):788-96. doi: 10.1038/ncb1604. Epub 2007 Jun 3. Nat Cell Biol. 2007. PMID: 17546040 - Structure and Function of the Nuclear Pore Complex Cytoplasmic mRNA Export Platform.
Fernandez-Martinez J, Kim SJ, Shi Y, Upla P, Pellarin R, Gagnon M, Chemmama IE, Wang J, Nudelman I, Zhang W, Williams R, Rice WJ, Stokes DL, Zenklusen D, Chait BT, Sali A, Rout MP. Fernandez-Martinez J, et al. Cell. 2016 Nov 17;167(5):1215-1228.e25. doi: 10.1016/j.cell.2016.10.028. Epub 2016 Nov 10. Cell. 2016. PMID: 27839866 Free PMC article. - [Nuclear pores: from yeast to higher eukaryotes].
Doye V. Doye V. J Soc Biol. 2002;196(4):349-54. J Soc Biol. 2002. PMID: 12645306 Review. French. - Toward the atomic structure of the nuclear pore complex: when top down meets bottom up.
Hoelz A, Glavy JS, Beck M. Hoelz A, et al. Nat Struct Mol Biol. 2016 Jul;23(7):624-30. doi: 10.1038/nsmb.3244. Epub 2016 Jun 6. Nat Struct Mol Biol. 2016. PMID: 27273515 Free PMC article. Review.
Cited by
- 8 Å structure of the outer rings of the Xenopus laevis nuclear pore complex obtained by cryo-EM and AI.
Tai L, Zhu Y, Ren H, Huang X, Zhang C, Sun F. Tai L, et al. Protein Cell. 2022 Oct;13(10):760-777. doi: 10.1007/s13238-021-00895-y. Epub 2022 Jan 11. Protein Cell. 2022. PMID: 35015240 Free PMC article. - IDPs in macromolecular complexes: the roles of multivalent interactions in diverse assemblies.
Fung HYJ, Birol M, Rhoades E. Fung HYJ, et al. Curr Opin Struct Biol. 2018 Apr;49:36-43. doi: 10.1016/j.sbi.2017.12.007. Epub 2018 Jan 4. Curr Opin Struct Biol. 2018. PMID: 29306779 Free PMC article. Review. - One Ring to Rule them All? Structural and Functional Diversity in the Nuclear Pore Complex.
Fernandez-Martinez J, Rout MP. Fernandez-Martinez J, et al. Trends Biochem Sci. 2021 Jul;46(7):595-607. doi: 10.1016/j.tibs.2021.01.003. Epub 2021 Feb 6. Trends Biochem Sci. 2021. PMID: 33563541 Free PMC article. Review. - Molecular basis of tRNA recognition by the Elongator complex.
Dauden MI, Jaciuk M, Weis F, Lin TY, Kleindienst C, Abbassi NEH, Khatter H, Krutyhołowa R, Breunig KD, Kosinski J, Müller CW, Glatt S. Dauden MI, et al. Sci Adv. 2019 Jul 10;5(7):eaaw2326. doi: 10.1126/sciadv.aaw2326. eCollection 2019 Jul. Sci Adv. 2019. PMID: 31309145 Free PMC article. - The Anchored Flexibility Model in LC8 Motif Recognition: Insights from the Chica Complex.
Clark S, Nyarko A, Löhr F, Karplus PA, Barbar E. Clark S, et al. Biochemistry. 2016 Jan 12;55(1):199-209. doi: 10.1021/acs.biochem.5b01099. Epub 2015 Dec 22. Biochemistry. 2016. PMID: 26652654 Free PMC article.
References
- Bailer S.M., Balduf C., and Hurt E.. 2001. The Nsp1p carboxy-terminal domain is organized into functionally distinct coiled-coil regions required for assembly of nucleoporin subcomplexes and nucleocytoplasmic transport. Mol. Cell. Biol. 21:7944–7955 10.1128/MCB.21.23.7944-7955.2001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases