Protein quality control and metabolism: bidirectional control in the heart - PubMed (original) (raw)
Review
Protein quality control and metabolism: bidirectional control in the heart
Zhao V Wang et al. Cell Metab. 2015.
Abstract
The prevalence of heart disease, especially heart failure, continues to increase, and cardiovascular disease remains the leading cause of death worldwide. As cardiomyocytes are essentially irreplaceable, protein quality control is pivotal to cellular homeostasis and, ultimately, cardiac performance. Three evolutionarily conserved mechanisms-autophagy, the unfolded protein response, and the ubiquitin-proteasome system-act in concert to degrade misfolded proteins and eliminate defective organelles. Recent advances have revealed that these mechanisms are intimately associated with cellular metabolism. Going forward, comprehensive understanding of the role of protein quality control mechanisms in cardiac pathology will require integration of metabolic pathways and metabolic control.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest
We declare no conflicts of interest.
Figures
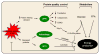
Figure 1. Protein quality control and cardiac metabolism in heart disease
Disease-related stress on the myocardium triggers increased demand for protein folding and protein damage. These events, in turn, activate the UPR, autophagy, and the UPS. Glucose and free fatty acid (FFA) are the major nutrients supporting energy production in the myocardium. Recent insights have uncovered complex interplay between these two major cellular processing, including bidirectional signaling, transcriptional control, and substrate provision.
Figure 2. The autophagy pathway
Autophagy is an evolutionarily conserved self-eating process. Autophagy is dynamic, involving multiple steps of initiation, vesicle nucleation, elongation/expansion and fusion. Engulfed cargo is digested by acid hydrolases from the lysosome, and degraded components are released into the cytosol to meet energetic demand. Several complexes participate in autophagosome formation at various steps, including an initiation complex, the class III PI3K complex, and two ubiquitin-like systems.
Figure 3. The unfolded protein response
The unfolded protein response (UPR) is an adaptive mechanism to cope with protein folding stress in the endoplasmic reticulum (ER). Misfolded proteins in the ER occupy the ER-resident chaperone BiP. Three signal transducers of the UPR are induced by a variety of mechanisms to attenuate global protein translation, and stimulate expression of genes coding for proteins involved in protein chaperoning, autophagy, ER-associated protein degradation, and metabolic regulation. In the setting of unremitting stress, cell death ensues to eliminate terminally defective cells. nATF6, nuclear ATF6.
Similar articles
- The critical roles of protein quality control systems in the pathogenesis of heart failure.
Maejima Y. Maejima Y. J Cardiol. 2020 Mar;75(3):219-227. doi: 10.1016/j.jjcc.2019.09.019. Epub 2019 Nov 4. J Cardiol. 2020. PMID: 31699567 Review. - The bitter end: the ubiquitin-proteasome system and cardiac dysfunction.
Patterson C, Ike C, Willis PW 4th, Stouffer GA, Willis MS. Patterson C, et al. Circulation. 2007 Mar 20;115(11):1456-63. doi: 10.1161/CIRCULATIONAHA.106.649863. Circulation. 2007. PMID: 17372187 Review. - Recent progress in research on molecular mechanisms of autophagy in the heart.
Maejima Y, Chen Y, Isobe M, Gustafsson ÅB, Kitsis RN, Sadoshima J. Maejima Y, et al. Am J Physiol Heart Circ Physiol. 2015 Feb 15;308(4):H259-68. doi: 10.1152/ajpheart.00711.2014. Epub 2014 Nov 14. Am J Physiol Heart Circ Physiol. 2015. PMID: 25398984 Free PMC article. Review. - Cardiac ubiquitin ligases: Their role in cardiac metabolism, autophagy, cardioprotection and therapeutic potential.
Parry TL, Willis MS. Parry TL, et al. Biochim Biophys Acta. 2016 Dec;1862(12):2259-2269. doi: 10.1016/j.bbadis.2016.07.002. Epub 2016 Jul 13. Biochim Biophys Acta. 2016. PMID: 27421947 Free PMC article. Review. - Into the heart: the emerging role of the ubiquitin-proteasome system.
Willis MS, Patterson C. Willis MS, et al. J Mol Cell Cardiol. 2006 Oct;41(4):567-79. doi: 10.1016/j.yjmcc.2006.07.015. Epub 2006 Sep 1. J Mol Cell Cardiol. 2006. PMID: 16949602 Review.
Cited by
- Ovarian Tumor Domain-Containing 7B Attenuates Pathological Cardiac Hypertrophy by Inhibiting Ubiquitination and Degradation of Krüppel-Like Factor 4.
Du BB, Zhang JL, Kong LY, Shi HT, Zhang DH, Wang X, Yang CL, Li PC, Yao R, Liang C, Wu LM, Huang Z. Du BB, et al. J Am Heart Assoc. 2023 Dec 19;12(24):e029745. doi: 10.1161/JAHA.123.029745. Epub 2023 Dec 12. J Am Heart Assoc. 2023. PMID: 38084712 Free PMC article. - Aging and Autophagy in the Heart.
Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK, Sadoshima J. Shirakabe A, et al. Circ Res. 2016 May 13;118(10):1563-76. doi: 10.1161/CIRCRESAHA.116.307474. Circ Res. 2016. PMID: 27174950 Free PMC article. Review. - Protein Folding and Mechanisms of Proteostasis.
Díaz-Villanueva JF, Díaz-Molina R, García-González V. Díaz-Villanueva JF, et al. Int J Mol Sci. 2015 Jul 28;16(8):17193-230. doi: 10.3390/ijms160817193. Int J Mol Sci. 2015. PMID: 26225966 Free PMC article. Review. - E3-ligase knock down revealed differential titin degradation by autopagy and the ubiquitin proteasome system.
Müller E, Salcan S, Bongardt S, Barbosa DM, Krüger M, Kötter S. Müller E, et al. Sci Rep. 2021 Oct 26;11(1):21134. doi: 10.1038/s41598-021-00618-7. Sci Rep. 2021. PMID: 34702928 Free PMC article. - Inflammation as a therapeutic target in heart failure with preserved ejection fraction.
Peh ZH, Dihoum A, Hutton D, Arthur JSC, Rena G, Khan F, Lang CC, Mordi IR. Peh ZH, et al. Front Cardiovasc Med. 2023 Jun 29;10:1125687. doi: 10.3389/fcvm.2023.1125687. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37456816 Free PMC article. Review.
References
- Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–750. - PubMed
- Antos CL, McKinsey TA, Dreitz M, Hollingsworth LM, Zhang CL, Schreiber K, Rindt H, Gorczynski RJ, Olson EN. Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. The Journal of biological chemistry. 2003;278:28930–28937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL100401/HL/NHLBI NIH HHS/United States
- HL-100401/HL/NHLBI NIH HHS/United States
- R01 HL080144/HL/NHLBI NIH HHS/United States
- R01 HL120732/HL/NHLBI NIH HHS/United States
- R01 HL090842/HL/NHLBI NIH HHS/United States
- HL-120732/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials