The contribution of PA-X to the virulence of pandemic 2009 H1N1 and highly pathogenic H5N1 avian influenza viruses - PubMed (original) (raw)
Yipeng Sun 1, Jiao Hu 2, Lu Qi 1, Jinliang Wang 1, Xin Xiong 1, Yu Wang 1, Qiming He 1, Yang Lin 1, Weili Kong 1, Lai-Giea Seng 3, Honglei Sun 1, Juan Pu 1, Kin-Chow Chang 3, Xiufan Liu 2, Jinhua Liu 1
Affiliations
- PMID: 25652161
- PMCID: PMC4317690
- DOI: 10.1038/srep08262
The contribution of PA-X to the virulence of pandemic 2009 H1N1 and highly pathogenic H5N1 avian influenza viruses
Huijie Gao et al. Sci Rep. 2015.
Abstract
PA-X is a novel protein encoded by PA mRNA and is found to decrease the pathogenicity of pandemic 1918 H1N1 virus in mice. However, the importance of PA-X proteins in current epidemiologically important influenza A virus strains is not known. In this study, we report on the pathogenicity and pathological effects of PA-X deficient 2009 pandemic H1N1 (pH1N1) and highly pathogenic avian influenza H5N1 viruses. We found that loss of PA-X expression in pH1N1 and H5N1 viruses increased viral replication and apoptosis in A549 cells and increased virulence and host inflammatory response in mice. In addition, PA-X deficient pH1N1 and H5N1 viruses up-regulated PA mRNA and protein synthesis and increased viral polymerase activity. Loss of PA-X was also accompanied by accelerated nuclear accumulation of PA protein and reduced suppression of PA on non-viral protein expression. Our study highlights the effects of PA-X on the moderation of viral pathogenesis and pathogenicity.
Figures
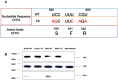
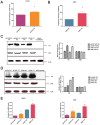
Figure 1. Generation of deficient PA-X in pH1N1 and HPAI H5N1 viruses.
(A) Mutations (U592A, C593G, U597C, C598A and U600A in red) introduced into the PA gene to abolish PA-X expression did not alter the PA ORF. (B) Western blot analysis for the detection of PA-X protein in MDCK cells infected with pH1N1and H5N1 mutant viruses (pH1N1-FS and H5N1-FS) showed absence of PA-X unlike corresponding pH1N1 WT and H5N1 WT viruses which expressed both PA and PA-X. Mock infection served as negative control. The samples of infection cells derived from the same experiment and the blots were processed in parallel. Given the expression level of PA-X protein much lower than PB1, PA and β actin, a long exposure was used for PA-X alone. Full-length blots are presented in Supplementary Figure 2.
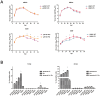
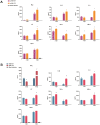
Figure 2. PA-X deficient viruses enhanced virus growth and apoptosis in A549 cells.
(A) Virus growth curves of PA-X mutant and WT viruses in MDCK cells and A549 cells over 84 h. (B) Relative induction of cell death as determined by the detection of annexin+, PI+ and annexin+ PI+ A549 cells infected with the panel of indicated viruses at a 1.0 MOI for 12 and 24 h. * indicates significant difference between viruses with PA-X mutations and corresponding wild type viruses. Each value represents the mean of three independent experiments performed in triplicates; error bars indicate standard deviations. Differences were considered statistically significant at P < 0.05.
Figure 3. Mortality and weight loss comparisons of mice intranasally inoculated with pH1N1 (A) and H5N1 (B) viruses (PA-X deficient and wild type).
Data show the survival (percentage) of mice (3 per group) infected with PA-X deficient and wild type viruses. The body weight of mice inoculated with PA-X mutant viruses was presented as percentage of the weight on the day of inoculation (day 0). Any mouse that lost more than 30% of its body weight was euthanized. The means of each group are shown, and error bars are SDs. * indicates significant difference between PA-X deficient virus and corresponding wild type virus. Differences were considered statistically significant at P < 0.05.
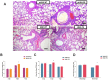
Figure 4. Histopathological changes (A) and virus titers in lungs (B and C) or in brains and blood (D) of mice infected with pH1N1 and H5N1 viruses (PA-X deficient and wild type).
PA-X deficient pH1N1 and H5N1 viruses conferred more severe edema and inflammatory consolidation than corresponding WT viruses (A). Scale bars, 100 μm. The mean viral lung load ± SD was calculated by log10 TCID50 determination in MDCK cells. * indicates significant difference between H1N1 WT and H1N1-FS viruses, or between H5N1 WT and H5N1-FS viruses. Differences were considered statistically significant at P < 0.05.
Figure 5. Detection of cytokine/chemokine proteins in lungs of mice infected with PA-X deficient and wild type pH1N1 (A) and H5N1 (B) viruses.
Mean cytokine/chemokine levels ± SD are shown. * indicates significant difference between wild type and corresponding PA-X deficient viruses. Differences were considered statistically significant at P < 0.05.
Figure 6. PA-X deficiency increased viral polymerase activity and expression of PA protein.
Polymerase activities of PA-X deficient and wild type pH1N1 (A) and H5N1 (B) viruses are expressed as mean percentage activity ± SD, with corresponding wild-type PA set at 100% from three independent experiments. Western blot analysis to detect PA, PB1 and β-actin in protein lysates from 293T cells transfected with polymerase plasmids (C) and from MDCK cells infected with PA-X deficient and wild type viruses at 1.0 MOI for 12 h (D). The protein bands were quantified by densitometry. Relative protein levels of PA or PB1 as compared with β-actin are shown as histograms. Mock transfection and mock infection served as negative control. The samples of infection or transfection cells derived from the same experiment and the blots were processed in parallel. Full-length blots are presented in Supplementary Figure 2. MDCK cells and A549 cells (E) were infected with the indicated viruses at 1.0 MOI for 12 h. PA mRNA expression was normalized to NP mRNA expression of the same virus. * indicates significant difference between virus with PA-X deficient and corresponding wild type virus. Differences were considered statistically significant at P < 0.05. The values shown are means ± SD.
Figure 7. Nuclear transport of mutant and wild type PA proteins in infected cells.
(A) PA protein in A549 cells infected with H1N1 WT, H1N1-FS, H5N1 WT or H5N1-FS virus at 2.0 MOI was localized by immunofluorescence at 2, 6, 8 and 12 hpi. Nuclei were stained with DAPI. (B) At 8 hpi, nuclear PA quantification was based on proportion of infected cells (n = 100) with clear nuclear presence of PA. Values shown are means of the results of three independent experiments ± SDs. *, P < 0.05 compared with corresponding wild type virus infected cells. Differences were considered statistically significant at P < 0.05.
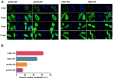
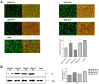
Figure 8. PAs were less able to suppress non-viral protein synthesis in the absence of PA-X. (A) 293T cells were co-transfected with eGFP expression plasmid and wild type or mutant PA plasmids of pH1N1 and H5N1 viruses.
(A) Fluorescence images indicative of eGFP expression at 24 h post-transfection were captured under identical exposure conditions. Control was 293T cells co-transfected with eGFP expression plasmid and empty pcDNA3.1 vector. Dark and half bright field images were shown. The GFP expression levels were quantified by counting GFP positive cells by fluorescence microscopy. Relative GFP expression levels of each group as compared with control are shown as histograms. (B) Expressed PA protein was determined by Western blot analysis using anti-PA antibody. Anti-β-actin antibody was used as a loading control. The protein bands were quantified by densitometry. Relative protein levels of PA as compared with β-actin are shown as histograms. Full-length blots are presented in Supplementary Figure 2. Note PA-X deficient PA plasmids conferred more PA protein expression. Differences were considered statistically significant at P < 0.05. The results shown are representative data from three independent experiments. Error bars indicate standard deviations.
Similar articles
- Caspase-1 deficient mice are more susceptible to influenza A virus infection with PA variation.
Huang CH, Chen CJ, Yen CT, Yu CP, Huang PN, Kuo RL, Lin SJ, Chang CK, Shih SR. Huang CH, et al. J Infect Dis. 2013 Dec 1;208(11):1898-905. doi: 10.1093/infdis/jit381. Epub 2013 Jul 30. J Infect Dis. 2013. PMID: 23901080 - PA-X decreases the pathogenicity of highly pathogenic H5N1 influenza A virus in avian species by inhibiting virus replication and host response.
Hu J, Mo Y, Wang X, Gu M, Hu Z, Zhong L, Wu Q, Hao X, Hu S, Liu W, Liu H, Liu X, Liu X. Hu J, et al. J Virol. 2015 Apr;89(8):4126-42. doi: 10.1128/JVI.02132-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631083 Free PMC article. - Functional Evolution of the 2009 Pandemic H1N1 Influenza Virus NS1 and PA in Humans.
Nogales A, Martinez-Sobrido L, Chiem K, Topham DJ, DeDiego ML. Nogales A, et al. J Virol. 2018 Sep 12;92(19):e01206-18. doi: 10.1128/JVI.01206-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021892 Free PMC article. - Virulence of pandemic (H1N1) 2009 influenza A polymerase reassortant viruses.
Song MS, Pascua PN, Choi YK. Song MS, et al. Virulence. 2011 Sep-Oct;2(5):422-6. doi: 10.4161/viru.2.5.17267. Epub 2011 Sep 1. Virulence. 2011. PMID: 21921678 Review. - Progress in identifying virulence determinants of the 1918 H1N1 and the Southeast Asian H5N1 influenza A viruses.
Basler CF, Aguilar PV. Basler CF, et al. Antiviral Res. 2008 Sep;79(3):166-78. doi: 10.1016/j.antiviral.2008.04.006. Epub 2008 May 23. Antiviral Res. 2008. PMID: 18547656 Free PMC article. Review.
Cited by
- Ribosomal frameshifting and transcriptional slippage: From genetic steganography and cryptography to adventitious use.
Atkins JF, Loughran G, Bhatt PR, Firth AE, Baranov PV. Atkins JF, et al. Nucleic Acids Res. 2016 Sep 6;44(15):7007-78. doi: 10.1093/nar/gkw530. Epub 2016 Jul 19. Nucleic Acids Res. 2016. PMID: 27436286 Free PMC article. Review. - Accessory Gene Products of Influenza A Virus.
Pinto RM, Lycett S, Gaunt E, Digard P. Pinto RM, et al. Cold Spring Harb Perspect Med. 2021 Dec 1;11(12):a038380. doi: 10.1101/cshperspect.a038380. Cold Spring Harb Perspect Med. 2021. PMID: 32988983 Free PMC article. Review. - PA-X-associated early alleviation of the acute lung injury contributes to the attenuation of a highly pathogenic H5N1 avian influenza virus in mice.
Hu J, Mo Y, Gao Z, Wang X, Gu M, Liang Y, Cheng X, Hu S, Liu W, Liu H, Chen S, Liu X, Peng D, Liu X. Hu J, et al. Med Microbiol Immunol. 2016 Aug;205(4):381-95. doi: 10.1007/s00430-016-0461-2. Epub 2016 Jun 11. Med Microbiol Immunol. 2016. PMID: 27289459 Free PMC article. - Selective Degradation of Host RNA Polymerase II Transcripts by Influenza A Virus PA-X Host Shutoff Protein.
Khaperskyy DA, Schmaling S, Larkins-Ford J, McCormick C, Gaglia MM. Khaperskyy DA, et al. PLoS Pathog. 2016 Feb 5;12(2):e1005427. doi: 10.1371/journal.ppat.1005427. eCollection 2016 Feb. PLoS Pathog. 2016. PMID: 26849127 Free PMC article. - A Novel H1N2 Influenza Virus Related to the Classical and Human Influenza Viruses from Pigs in Southern China.
Song Y, Wu X, Wang N, Ouyang G, Qu N, Cui J, Qi Y, Liao M, Jiao P. Song Y, et al. Front Microbiol. 2016 Jul 8;7:1068. doi: 10.3389/fmicb.2016.01068. eCollection 2016. Front Microbiol. 2016. PMID: 27458456 Free PMC article.
References
- Palese P. The genes of influenza virus. Cell 10, 1 (1977). - PubMed
- Lamb R. The influenza virus RNA segments and their encoded proteins. Genetics of influenza viruses. [Palese, P. & Kingsbury, D. W. (eds).] 26–69 (Springer, New York, 1983).
- Chen W. et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med 7, 1306–1312 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials