Axon initial segment-associated microglia - PubMed (original) (raw)
Axon initial segment-associated microglia
Kelli Baalman et al. J Neurosci. 2015.
Abstract
Microglia are the brain's resident immune cells and function as the main defense against pathogens or injury. However, in the absence of disease, microglia have other functions in the normal brain. For example, previous studies showed that microglia contribute to circuit refinement and synaptic plasticity in the developing and adult brain, respectively. Thus, microglia actively participate in regulating neuronal excitability and function. Here, we report that in the cortex, but not other brain regions, a subset of microglia extend a single process that specifically associates and overlaps with the axon initial segment (AIS), the site where action potentials are generated. Similar associations were not observed with dendrites or distal axons. Microglia-AIS interactions appear early in development, persist throughout adulthood, and are conserved across species including mice, rats, and primates. However, these interactions are lost after microglial activation following brain injury, suggesting that such interactions may be part of healthy brain function. Loss of microglial CX3CR1 receptors, or the specialized extracellular matrix surrounding the AIS, did not disrupt the interaction. However, loss of AIS proteins by the neuron-specific deletion of the master AIS scaffold AnkyrinG disrupted microglia-AIS interactions. These results reveal a unique population of microglia that specifically interact with the AIS in the adult cortex.
Keywords: axon; axon initial segment; brain injury; glia; ion channel; microglia.
Copyright © 2015 the authors 0270-6474/15/352283-10$15.00/0.
Figures
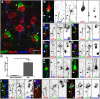
Figure 1.
A subset of satellite microglia interact with the AIS. A, Mouse cortical neurons labeled for NeuN (red) and AnkG (blue) are contacted by perineuronal satellite microglia (arrow; GFP from a CX3CR1GFP/+ mouse). B, A mouse cortical neuron labeled for NeuN (red) and AnkG (blue), and a perineuronal satellite microglia aligned along the AIS (arrow; GFP from a CX3CR1GFP/+ mouse). Hoechst labels nuclei (white in the merged image). C, Immunostaining for FGF14 to label the AIS (red, arrow), Iba1 to label microglia (green), and Hoechst to label nuclei (blue). D, Immunostaining for AnkG to label the AIS (red, arrow), Iba1 to label microglia (green), and Hoechst to label nuclei (blue). E, Immunostaining for Nav1.6 to label the AIS (red, arrow), Iba1 to label microglia (green), and Hoechst to label nuclei (blue). F, Immunostaining for βIV spectrin to label the AIS (green, arrow), Cd11b to label microglia (red), and Hoechst to label nuclei (blue). G, Quantification of associations between microglia and the apical dendrite or AIS in Thy1+ layer 5 pyramidal cells (n = 3, 904 cells). H, Satellite microglia do not associate with the apical dendrite (arrow) in layer 5 cells. I, Example of overlapping AIS (arrow) and microglial branch in Thy1+ cell. J–L, Immunostaining of AIS (AnkG, red), microglia (Iba1, green), and nuclei (Hoechst) in mouse (J), rat (K), and macaque (L) cortex. Scale bars: A, 10 μm; B–F, H_–_L, 5 μm. *p < 0.05 (unpaired t test with Welch's correction).
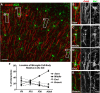
Figure 2.
AIS-associated microglial cell bodies are preferentially located at the start of the AIS. A, AnkG-labeled cortical AIS (red) and GFP+ microglia from a CX3CR1+/GFP mouse show multiple examples of AIS–microglia overlaps (white boxes). B–D, Microglial cell bodies located at the start (B), middle (C), and end (D) of the AIS. E, A “branched: microglial cell process associated with the AIS. F, Quantification of the location of the AIS-associated microglial cell body relative to the AIS at P9, P15, and P30 and in the adult. P9, P15, and P30 data are from M1 only; adult data include regions M1, S1BF, and V1. Scale bars: A, 25 μm; B–E, 8 μm.
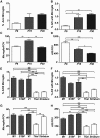
Figure 3.
The frequency and distribution of AXIS microglia. A, The percentage of AXIS microglia as a function of age. B, The percentage of AISs with an associated microglia as a function of age. C, The number of microglia per FOV changes as a function of age. D, The number of AISs per FOV decreases as the size of the brain increases during development. E, F, The percentage of AXIS microglia in different brain regions: M1, S1BF, V1, thalamus (Thal), and striatum. G, H, The numbers of microglia per FOV and AISs per FOV in different brain regions. *p < 0.05; **p < 0.01; ***p < 0.001 (one-way ANOVA with Bonferroni's multiple comparison test). n = 3 animals in all experiments.
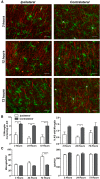
Figure 4.
Activated microglia do not associate with the AIS. A, Representative images from 3, 12, and 72 h after injury on both the injured and contralateral sides of the brain. Asterisks indicate AXIS microglia (green, anti-Iba1); AISs are labeled by anti-AnkG (red). B, The percentage of AXIS microglia at 3, 24, and 72 h after injury. C, The number of microglia is significantly higher 72 h after injury, whereas the number of AISs does not change. Scale bars, 25 μm. *p < 0.05; **p < 0.01; ***p < 0.001 (two-way ANOVA with Bonferroni multiple comparison test), n = 3 animals per time point.
Figure 5.
AXIS microglia preferentially interact with non-GABAergic neurons. A, Cortex showing GABAergic neurons (tdTomato, red), AISs (anti-AnkG, blue), and microglia (anti-Iba1, green). B, C, Example of a microglia/AIS interaction in a tdTomato+ cell (B) and a tdTomato− cell (C). Arrows show overlapping processes, and the asterisk marks the neuronal cell body. D, The percentage of tdTomato+ and tdTomato− neurons with AXIS microglia (n = 4 mice). Scale bars: A, 25 μm; B, C, 5 μm. *p < 0.05 (unpaired Student's t test).
Figure 6.
An intact AIS is required for AIS–microglia interactions. A, AXIS microglia in a CX3CR1GFP/GFP (CX3CR1 KO) mouse. The AIS (arrow) is labeled using anti-βIV spectrin antibodies (red). B, AXIS microglia labeled using Iba1 antibodies (red) in a Brevican−/−;Versican−/− (BcVc DKO) mouse. The AIS (arrow) is labeled using anti-AnkG antibodies (green). C, D, Neither the percentage of microglia contacting the AIS nor the percentage of AIS with AXIS microglia is different between control, CX3CR1 KO, and BcVc DKO mice. E, F, Neither the number of microglia per FOV nor the number of AISs per FOV is different between control, CX3CR1 KO, and BcVc DKO mice. G, A perineuronal satellite microglia (Iba1, red) at the start of the axon in a Cre-GFP electroporated, AnkG-deficient neuron. AISs are labeled using anti-AnkG antibodies (blue), and nuclei are labeled by Hoechst (white in the merged image). The axon is indicated by an arrow, and the microglial process does not interact with the axon. H, Example of an interaction in a neuron lacking AnkG with the microglia in the middle of the AIS. The axon is indicated by an arrow. I, Example of a control GFP+ cell (green) with an AXIS microglia labeled using anti-Iba1 antibodies (red). The AIS is labeled using antibodies against AnkG (blue), and the nuclei of the neuron and AXIS microglia are labeled using Hoechst (white in the merged image). J, K, The percentage of control and AnkG-deficient (AnkG KO) neurons with an AXIS microglia (J) or satellite microglia (K). Scale bars: A, B, 5 μm; G–I, 8 μm. **p < 0.01 (unpaired Student's t test). In A–F, n = 3 mice per genotype. In G–K, n = 3 mice for AnkG KO, with 1011 analyzed, and n = 4 mice for controls, with 1014 neurons analyzed.
Comment in
- Glia: Anchored at the axon.
Carr F. Carr F. Nat Rev Neurosci. 2015 Apr;16(4):186-7. doi: 10.1038/nrn3934. Epub 2015 Feb 25. Nat Rev Neurosci. 2015. PMID: 25712319 No abstract available.
Similar articles
- Axon Initial Segments Are Required for Efficient Motor Neuron Axon Regeneration and Functional Recovery of Synapses.
Teliska LH, Dalla Costa I, Sert O, Twiss JL, Rasband MN. Teliska LH, et al. J Neurosci. 2022 Oct 26;42(43):8054-8065. doi: 10.1523/JNEUROSCI.1261-22.2022. Epub 2022 Sep 12. J Neurosci. 2022. PMID: 36096668 Free PMC article. - Axonal injury alters the extracellular glial environment of the axon initial segment and allows substantial mitochondrial influx into axon initial segment.
Tamada H, Kiryu-Seo S, Sawada S, Kiyama H. Tamada H, et al. J Comp Neurol. 2021 Nov;529(16):3621-3632. doi: 10.1002/cne.25212. Epub 2021 Jul 16. J Comp Neurol. 2021. PMID: 34235750 - A unique ion channel clustering domain on the axon initial segment of mammalian neurons.
King AN, Manning CF, Trimmer JS. King AN, et al. J Comp Neurol. 2014 Aug 1;522(11):2594-608. doi: 10.1002/cne.23551. J Comp Neurol. 2014. PMID: 24477962 Free PMC article. - Axon initial segments: diverse and dynamic neuronal compartments.
Yoshimura T, Rasband MN. Yoshimura T, et al. Curr Opin Neurobiol. 2014 Aug;27:96-102. doi: 10.1016/j.conb.2014.03.004. Epub 2014 Apr 3. Curr Opin Neurobiol. 2014. PMID: 24705243 Free PMC article. Review. - Building and maintaining the axon initial segment.
Grubb MS, Burrone J. Grubb MS, et al. Curr Opin Neurobiol. 2010 Aug;20(4):481-8. doi: 10.1016/j.conb.2010.04.012. Epub 2010 May 27. Curr Opin Neurobiol. 2010. PMID: 20537529 Free PMC article. Review.
Cited by
- Microglia regulate chandelier cell axo-axonic synaptogenesis.
Gallo NB, Berisha A, Van Aelst L. Gallo NB, et al. Proc Natl Acad Sci U S A. 2022 Mar 15;119(11):e2114476119. doi: 10.1073/pnas.2114476119. Epub 2022 Mar 9. Proc Natl Acad Sci U S A. 2022. PMID: 35263225 Free PMC article. - Interactions Between Microglia and Newly Formed Hippocampal Neurons in Physiological and Seizure-Induced Inflammatory Environment.
Chugh D, Ekdahl CT. Chugh D, et al. Brain Plast. 2016 Jun 29;1(2):215-221. doi: 10.3233/BPL-150014. Brain Plast. 2016. PMID: 29765843 Free PMC article. - Priming the inflammatory pump of the CNS after traumatic brain injury.
Witcher KG, Eiferman DS, Godbout JP. Witcher KG, et al. Trends Neurosci. 2015 Oct;38(10):609-620. doi: 10.1016/j.tins.2015.08.002. Trends Neurosci. 2015. PMID: 26442695 Free PMC article. Review. - Three-dimensional ultrastructure analysis of organelles in injured motor neuron.
Tamada H. Tamada H. Anat Sci Int. 2023 Jul;98(3):360-369. doi: 10.1007/s12565-023-00720-y. Epub 2023 Apr 18. Anat Sci Int. 2023. PMID: 37071350 Free PMC article. Review. - Glial Regulation of Circuit Wiring, Firing, and Expiring in the Drosophila Central Nervous System.
Coutinho-Budd J, Freeman MR, Ackerman S. Coutinho-Budd J, et al. Cold Spring Harb Perspect Biol. 2024 Dec 2;16(12):a041347. doi: 10.1101/cshperspect.a041347. Cold Spring Harb Perspect Biol. 2024. PMID: 38565270 Free PMC article. Review.
References
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. - DOI - PMC - PubMed
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 NS044916/NS/NINDS NIH HHS/United States
- R01 NS044916/NS/NINDS NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- R01 NS069688/NS/NINDS NIH HHS/United States
- NS069688/NS/NINDS NIH HHS/United States
- NS044916/NS/NINDS NIH HHS/United States
- R25 GM069234/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases