Dissecting the mechanisms of the clock in Neurospora - PubMed (original) (raw)
Review
Dissecting the mechanisms of the clock in Neurospora
Jennifer Hurley et al. Methods Enzymol. 2015.
Abstract
The circadian clock exists to synchronize inner physiology with the external world, allowing life to anticipate and adapt to the continual changes that occur in an organism's environment. The clock architecture is highly conserved, present in almost all major branches of life. Within eukaryotes, the filamentous fungus Neurospora crassa has consistently been used as an excellent model organism to uncover the basic circadian physiology and molecular biology. The Neurospora model has elucidated our fundamental understanding of the clock as nested positive and negative feedback loop, regulated by transcriptional and posttranscriptional processes. This review will examine the basics of circadian rhythms in the model filamentous fungus N. crassa as well as highlight the output of the clock in Neurospora and the reasons that N. crassa has continued to be a strong model for the study of circadian rhythms. It will also synopsize classical and emerging methods in the study of the circadian clock.
Keywords: Circadian; Frequency; Molecular clock; Neurospora crassa; Output; White Collar Complex.
© 2015 Elsevier Inc. All rights reserved.
Figures
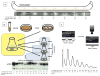
Figure 1
Methods of circadian analysis in Neurospora. (A) The basic outline of the use of a race tube in analyzing circadian rhythms in Neurospora along with the image of an actual race tube beneath it. Daily growth fronts are noted with vertical lines (sidereal time and in circadian time are noted below; J. Hurley, unpublished data). (B) The basics of analysis of the molecular rhythms for Neurospora liquid culture. The outline of the protocol to extract either mRNA or protein from Neurospora over circadian time. Western blot of FRQ protein tracked over 48 sidereal hours (time points taken every four sidereal hours) and labeled in circadian hours, highlighting the changes of phosphorylation state of FRQ protein over time (J. Emerson, unpublished data). (C) The outline of real-time analysis of molecular circadian rhythms. Ninety-six individual tubes of Neurospora are subjected to a 12:12 light/dark cycle and then allowed to free run in the dark. A luciferase trace of frq mRNA expression tracked over 144 sidereal hours using a CCD camera and the resulting traces are labeled in circadian hours, highlighting the changes of expression levels of frq mRNA over time (J. Emerson, unpublished data).
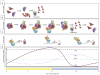
Figure 2
Neurospora circadian cycle at the molecular level. (A) If FRQ is not able to bind to its stabilizer, FRH, it is degraded by default due to the inherently disordered nature of FRQ and is unable to complete its function in the circadian clock. (B) During the late subjective night of the circadian cycle, the WCC induces expression of frq mRNA, leading to a rapid increase in FRQ translation. FRQ forms a homodimer and binds to its stabilizer FRH, allowing for the IDP FRQ to avoid degradation by default. As the circadian day progresses, FRQ is phosphorylated via interaction with several kinases. FRQ inhibits the activity of the WCC by promoting the phosphorylation of the WCC, turning off frq transcription. FRQ levels decrease as no new FRQ is made while old FRQ is increasingly phosphorylated, which leads to ubiquitination facilitated by FWD-1, leading to FRQ degradation. (C) Factors that drive the output of the circadian clock. Low FRQ levels cause WCC activity to increase which subsequently leads to the expression of frq mRNA as well as mRNAs from other _ccg_s. FRQ binds to the WCC, promoting phosphorylation of the WCC and causing the WCC to become inactive. Decreasing FRQ levels allow phosphatases to bind the WCC, dephosphorylating the WCC, and increasing WCC activation. (D) Protein levels of the core clock components. While FRH and WC-2 remain constant, FRQ and WC-1 oscillate in opposite phases to one another. Stars represent phosphorylation and lightning bolts represent ubiquitination.
Similar articles
- The circadian clock of Neurospora crassa.
Baker CL, Loros JJ, Dunlap JC. Baker CL, et al. FEMS Microbiol Rev. 2012 Jan;36(1):95-110. doi: 10.1111/j.1574-6976.2011.00288.x. Epub 2011 Aug 1. FEMS Microbiol Rev. 2012. PMID: 21707668 Free PMC article. Review. - Interlocked feedback loops of the circadian clock of Neurospora crassa.
Brunner M, Káldi K. Brunner M, et al. Mol Microbiol. 2008 Apr;68(2):255-62. doi: 10.1111/j.1365-2958.2008.06148.x. Epub 2008 Feb 26. Mol Microbiol. 2008. PMID: 18312266 Review. - Methods to study molecular mechanisms of the Neurospora circadian clock.
Cha J, Zhou M, Liu Y. Cha J, et al. Methods Enzymol. 2015;551:137-51. doi: 10.1016/bs.mie.2014.10.002. Epub 2014 Dec 26. Methods Enzymol. 2015. PMID: 25662455 Free PMC article. - Mechanism of the Neurospora circadian clock, a FREQUENCY-centric view.
Cha J, Zhou M, Liu Y. Cha J, et al. Biochemistry. 2015 Jan 20;54(2):150-6. doi: 10.1021/bi5005624. Epub 2014 Dec 30. Biochemistry. 2015. PMID: 25302868 Free PMC article. Review. - A fungus among us: the Neurospora crassa circadian system.
Merrow M, Roenneberg T, Macino G, Franchi L. Merrow M, et al. Semin Cell Dev Biol. 2001 Aug;12(4):279-85. doi: 10.1006/scdb.2001.0255. Semin Cell Dev Biol. 2001. PMID: 11463212 Review.
Cited by
- The circadian system as an organizer of metabolism.
Hurley JM, Loros JJ, Dunlap JC. Hurley JM, et al. Fungal Genet Biol. 2016 May;90:39-43. doi: 10.1016/j.fgb.2015.10.002. Epub 2015 Oct 20. Fungal Genet Biol. 2016. PMID: 26498192 Free PMC article. Review. - An Anatomy of Fungal Eye: Fungal Photoreceptors and Signalling Mechanisms.
Bayram ÖS, Bayram Ö. Bayram ÖS, et al. J Fungi (Basel). 2023 May 19;9(5):591. doi: 10.3390/jof9050591. J Fungi (Basel). 2023. PMID: 37233302 Free PMC article. Review. - The Resonance and Adaptation of Neurospora crassa Circadian and Conidiation Rhyth ms to Short Light-Dark Cycles.
Ma H, Li L, Yan J, Zhang Y, Ma X, Li Y, Yuan Y, Yang X, Yang L, Guo J. Ma H, et al. J Fungi (Basel). 2021 Dec 29;8(1):27. doi: 10.3390/jof8010027. J Fungi (Basel). 2021. PMID: 35049967 Free PMC article. - Making Time: Conservation of Biological Clocks from Fungi to Animals.
Dunlap JC, Loros JJ. Dunlap JC, et al. Microbiol Spectr. 2017 May;5(3):10.1128/microbiolspec.funk-0039-2016. doi: 10.1128/microbiolspec.FUNK-0039-2016. Microbiol Spectr. 2017. PMID: 28527179 Free PMC article. Review. - Establishment of Neurospora crassa as a host for heterologous protein production using a human antibody fragment as a model product.
Havlik D, Brandt U, Bohle K, Fleißner A. Havlik D, et al. Microb Cell Fact. 2017 Jul 25;16(1):128. doi: 10.1186/s12934-017-0734-5. Microb Cell Fact. 2017. PMID: 28743272 Free PMC article.
References
- Aronson BD, Johnson KA, Loros JJ, Dunlap JC. Negative feedback defining a circadian clock: Autoregulation of the clock gene frequency. Science. 1994;263:1578–1584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources