Who is the puppet master? Replication of a parasitic wasp-associated virus correlates with host behaviour manipulation - PubMed (original) (raw)
. 2015 Mar 22;282(1803):20142773.
doi: 10.1098/rspb.2014.2773.
Fanny Maure 2, Marc Ravallec 3, Richard Galinier 4, Josée Doyon 5, David Duval 4, Lucas Leger 6, Anne-Nathalie Volkoff 3, Dorothée Missé 6, Sabine Nidelet 7, Vincent Demolombe 7, Jacques Brodeur 5, Benjamin Gourbal 4, Frédéric Thomas 6, Guillaume Mitta 4
Affiliations
- PMID: 25673681
- PMCID: PMC4345448
- DOI: 10.1098/rspb.2014.2773
Who is the puppet master? Replication of a parasitic wasp-associated virus correlates with host behaviour manipulation
Nolwenn M Dheilly et al. Proc Biol Sci. 2015.
Abstract
Many parasites modify their host behaviour to improve their own transmission and survival, but the proximate mechanisms remain poorly understood. An original model consists of the parasitoid Dinocampus coccinellae and its coccinellid host, Coleomegilla maculata; during the behaviour manipulation, the parasitoid is not in contact with its host anymore. We report herein the discovery and characterization of a new RNA virus of the parasitoid (D. coccinellae paralysis virus, DcPV). Using a combination of RT-qPCR and transmission electron microscopy, we demonstrate that DcPV is stored in the oviduct of parasitoid females, replicates in parasitoid larvae and is transmitted to the host during larval development. Next, DcPV replication in the host's nervous tissue induces a severe neuropathy and antiviral immune response that correlate with the paralytic symptoms characterizing the behaviour manipulation. Remarkably, virus clearance correlates with recovery of normal coccinellid behaviour. These results provide evidence that changes in ladybeetle behaviour most likely result from DcPV replication in the cerebral ganglia rather than by manipulation by the parasitoid. This offers stimulating prospects for research on parasitic manipulation by suggesting for the first time that behaviour manipulation could be symbiont-mediated.
Keywords: behavioural manipulation; holobiont; host–parasite interaction; parasitoid wasp; symbiont; virus.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures
Figure 1.
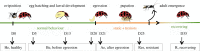
Life cycle of the parasitoid exploiting its host (drawing by Franz Vanoosthuyse). Boxes indicate when the samples were collected for analyses: healthy ladybeetle (He), before parasitoid larval egression (Be), after parasitoid larval egression (Ae), resistant ladybeetle (Res) and following host recovery (R).
Figure 2.
Schematic diagram of the predicted DcPV genome structure (electronic supplementary material, Note S2). Numbers on the top indicate nucleotide positions, numbers on the bottom indicate amino acid positions, and the long shaded box represents the single ORF. A leader sequence was found upstream of the viral capsid proteins (VP). Predicted proteins are indicated using the L434 nomenclature system. Boxes indicate the position of recognizable protein domains of structural proteins (VP 1 to 4; open boxes) and non-structural proteins (dark boxes).
Figure 3.
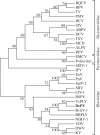
DcPV belongs to the Iflaviridae family. A phylogenetic tree was constructed from the alignment of 30 RdRp sequences (electronic supplementary material, table S3) by using the maximum-likelihood method (bootstrap on 1000 replicates). Branch lengths are proportional to the number of changes.
Figure 4.
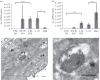
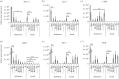
Abundance and replication of DcPV in D. coccinellae. Quantity of negative- (a) and positive- (b) strand copies of DcPV in 500 μg of RNA from parasitoid eggs (E) and larvae (L) collected 5, 13 and 20 days following oviposition (E Be D5, E/L Be D13, L Be D20), immediately after larval egression from the host (L Ae) and in adult parasitoids. Results are mean ± s.e.m. of biological replicates. Asterisks (*, ** and ***) indicate results are significantly different for a two-sided Student's _t_-test (electronic supplementary material, Note S3), with q < 0.05, q < 0.01 and q < 0.001, respectively. (c) TEM image of the oviduct of D. coccinellae. Beneath the cuticular intima, a series of microvilli line the lumen. Viral particles are observed within unilamellar vesicles. (d) TEM image of a vesicle packed with viruses showing a typical crystal structure. Cu, cuticular intima lining the oviductal lumen; mv, microvilli; L, lumen; arrow heads, viral particles. Scale bars, 500 nm.
Figure 5.
Abundance and replication of DcPV in healthy and parasitized C. maculata. Quantity of negative- (a) and positive- (b) strand copies of DcPV in 500 μg of RNA from abdomens and heads of ladybeetles collected healthy (He), 5, 13 and 20 days post-oviposition (Be D5, Be D13 and Be D20), immediately after larval egression (Ae), during recovery of a normal behaviour (R) and in resistant ladybeetles (Res). Results are mean ± s.e.m. of biological replicates. Asterisks (*, ** and ***) indicate that results are significantly different for a two-sided Student's _t_-test (electronic supplementary material, Note S3) with q < 0.05, q < 0.01 and q < 0.001, respectively.
Figure 6.
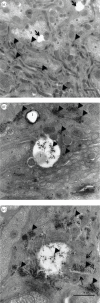
Ultrastructure of the neuropile of parasitized ladybeetles before larval egression. Virus particles were abundant in the cytoplasm of glial cells. (a,c) Numerous vacuoles formed within the neuropile. (b) Viruses were always abundant around lipid droplets and (c) in vacuoles that formed within glial cells. (c) Multilamellar structures were seen surrounding virus particles. a, axon; m, mitochondria; g, glia; t, trachea; l, lipid droplet; arrow heads, virus particles; black arrows, vacuoles; thin arrows, multilamellar structures. Scale bars, 500 nm.
Figure 7.
Ultrastructure of the neuropile of ladybeetles after larval egression and onset of the bodyguard behaviour. (a) Samples were characterized by a marked vacuolization of glial cells and (b) axon swelling. (c) Fingerprint-like structures were numerous within axons. (d) Autophagolysosomes were found within the cell soma of neurons. a, axon; a*, axon swelling, l, lipid droplet; t, trachea; black arrows, vacuoles; thick arrows, fingerprint-like structures; large arrows, phagosomes.
Figure 8.
Ultrastructure of the neuropile of parasitized ladybeetles that recovered from bodyguard manipulation. (a,b) Glia expanded between the cortex and neuropile, and (c,d) surrounded axons and axon bundles. a, axon; n, nucleus; m, mitochondria; thick arrows, expanding glial cells.
Figure 9.
Gene expression profiles of selected genes involved in antiviral immune response as obtained by RT-qPCR analysis: gene expression profiles in abdomens and heads of ladybeetles collected healthy (He), 5, 13 and 20 days post-oviposition (Be D5, Be D13 and Be D20), immediately after larval egression (Ae), during recovery of a normal behaviour (R) and in resistant ladybeetles (Res). (a) Dicer 2; (b) Ago2, Argonaute 2; (c) C3PO; (d) R2D2 are involved in antiviral RNA interference. (e) Toll 7 and (f) PI3K, phosphatidylinositol 3 kinase are involved in antiviral autophagosis. Results are means ± s.d. of biological replicates for each experimental condition. Asterisks (*, ** and ***) indicate results are significantly different to He for a two-sided student's _t_-test (electronic supplementary material, Note S3) with q < 0.05, q < 0.01 and q < 0.001, respectively.
Figure 10.
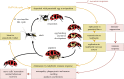
Life cycles of the parasitoid D. coccinellae and its endosymbiotic virus (D. coccinellae paralysis virus, DcPV) together with responses to parasitism and infection of the ladybeetle host C. maculata (drawing by Franz Vanoosthuyse). The DcPV is stored in abundance in the oviduct of D. coccinellae female. Following oviposition and egg hatching, DcPV replicates in the parasitoid larva and is transmitted to C. maculata. The antiviral immune system of the ladybeetle is then suppressed, which allows DcPV to replicate in glial cells in the host's nervous system. The re-establishment of the antiviral immune response correlates with a severe neuropathy in the ladybeetle and the onset of the bodyguard behaviour. The synchronized egression of the larva allows it to take advantage of the paralyzed ladybeetle: it steals between the ladybeetle legs and spins its cocoon under its protection. The DcPV is being eliminated from the ladybeetle, which progressively recovers through nerve cell restoration. By then, the parasitoid resumes pupation and emerges with a new load of DcPV in its oviduct.
Similar articles
- Bodyguard manipulation in a multipredator context: different processes, same effect.
Maure F, Brodeur J, Droit A, Doyon J, Thomas F. Maure F, et al. Behav Processes. 2013 Oct;99:81-6. doi: 10.1016/j.beproc.2013.06.003. Epub 2013 Jun 17. Behav Processes. 2013. PMID: 23791577 - Host behaviour manipulation as an evolutionary route towards attenuation of parasitoid virulence.
Maure F, Doyon J, Thomas F, Brodeur J. Maure F, et al. J Evol Biol. 2014 Dec;27(12):2871-5. doi: 10.1111/jeb.12530. Epub 2014 Nov 16. J Evol Biol. 2014. PMID: 25399504 - What can parasitoid wasps teach us about decision-making in insects?
Libersat F, Gal R. Libersat F, et al. J Exp Biol. 2013 Jan 1;216(Pt 1):47-55. doi: 10.1242/jeb.073999. J Exp Biol. 2013. PMID: 23225867 Review. - Wasp parasitoid disruption of host development: implications for new biologically based strategies for insect control.
Beckage NE, Gelman DB. Beckage NE, et al. Annu Rev Entomol. 2004;49:299-330. doi: 10.1146/annurev.ento.49.061802.123324. Annu Rev Entomol. 2004. PMID: 14651466 Review.
Cited by
- The genome sequence of a braconid wasp, Dinocampus coccinellae (Schrank, 1802).
Barclay MVL, Broad GR; Natural History Museum Genome Acquisition Lab; Darwin Tree of Life Barcoding collective; Wellcome Sanger Institute Tree of Life Management, Samples and Laboratory team; Wellcome Sanger Institute Scientific Operations: Sequencing Operations; Wellcome Sanger Institute Tree of Life Core Informatics team; Tree of Life Core Informatics collective; Darwin Tree of Life Consortium. Barclay MVL, et al. Wellcome Open Res. 2024 Aug 12;9:461. doi: 10.12688/wellcomeopenres.22862.1. eCollection 2024. Wellcome Open Res. 2024. PMID: 39391068 Free PMC article. - Drosophila are hosts to the first described parasitoid wasp of adult flies.
Moore LD, Chris Amuwa T, Shaw SR, Ballinger MJ. Moore LD, et al. Nature. 2024 Sep;633(8031):840-847. doi: 10.1038/s41586-024-07919-7. Epub 2024 Sep 11. Nature. 2024. PMID: 39261731 Free PMC article. - Body snatchers: these parasitoid wasps grow in adult fruit flies.
[No authors listed] [No authors listed] Nature. 2024 Sep 11. doi: 10.1038/d41586-024-02929-x. Online ahead of print. Nature. 2024. PMID: 39261688 No abstract available. - Entomophthovirus: an insect-derived iflavirus that infects a behavior-manipulating fungal pathogen of dipterans.
Coyle MC, Elya CN, Bronski MJ, Eisen MB. Coyle MC, et al. G3 (Bethesda). 2024 Oct 7;14(10):jkae198. doi: 10.1093/g3journal/jkae198. G3 (Bethesda). 2024. PMID: 39158097 Free PMC article. - The multispecies stinkbug iflavirus Halyomorpha halys virus detected in the multispecies stinkbug egg parasitoid microwasp, Telenomus podisi (Ashmead) (Hymenoptera: Platygastridae).
Dos Santos ER, de Camargo BR, da Silva LA, Laumann RA, Ribeiro BM, Ardisson-Araújo DMP. Dos Santos ER, et al. Braz J Microbiol. 2024 Jun;55(2):1913-1921. doi: 10.1007/s42770-024-01340-y. Epub 2024 Apr 14. Braz J Microbiol. 2024. PMID: 38615311
References
- Hughes DP, Brodeur J, Thomas F. 2012. Host manipulation by parasites, p. 224 Oxford, UK: Oxford University Press.
- Moore J. 2002. Parasites and the behavior of animals, p. 315 Oxford, UK: Oxford University Press.
- Poulin R. 2010. Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Stud. Behav. 41, 151–185. (10.1016/S0065-3454(10)41005-0) - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources