The S-nitrosylation status of PCNA localized in cytosol impacts the apoptotic pathway in a Parkinson's disease paradigm - PubMed (original) (raw)
The S-nitrosylation status of PCNA localized in cytosol impacts the apoptotic pathway in a Parkinson's disease paradigm
Liang Yin et al. PLoS One. 2015.
Abstract
It is generally accepted that nitric oxide (NO) or its derivatives, reactive nitrogen species (RNS), are involved in the development of Parkinson's disease (PD). Recently, emerging evidence in the study of PD has indicated that protein S-nitrosylation triggers the signaling changes in neurons. In this study, SH-SY5Y cells treated with rotenone were used as a model of neuronal death in PD. The treated cells underwent significant apoptosis, which was accompanied by an increase in intracellular NO in a rotenone dose-dependent manner. The CyDye switch approach was employed to screen for changes in S-nitrosylated (SNO) proteins in response to the rotenone treatment. Seven proteins with increased S-nitrosylation were identified in the treated SH-SY5Y cells, which included proliferating cell nuclear antigen (PCNA). Although PCNA is generally located in the nucleus and participates in DNA replication and repair, significant PCNA was identified in the SH-SY5Y cytosol. Using immunoprecipitation and pull-down approaches, PCNA was found to interact with caspase-9; using mass spectrometry, the two cysteine residues PCNA-Cys81 and -Cys162 were identified as candidate S-nitrosylated residues. In addition, the evidence obtained from in vitro and the cell model studies indicated that the S-nitrosylation of PCNA-Cys81 affected the interaction between PCNA and caspase-9. Furthermore, the interaction of PCNA and caspase-9 partially blocked caspase-9 activation, indicating that the S-nitrosylation of cytosolic PCNA may be a mediator of the apoptotic pathway.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
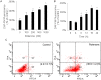
Fig 1. Phenotype changes in SH-SY5Y cells in response to rotenone treatment.
The dose (A) and time (B) responses of NO generated in SH-SY5Y cells that were treated with rotenone were analyzed. 18h treatment (A) and 500nM rotenone (B) were used for the gradient experiments. The NO contents are represented as the ratio of the intensity of DAF-FM fluorescence in the rotenone-treated group compared with the vehicle-treated group (average ratio ± SEM, n = 3, *P<0.05). Apoptosis assessment by flow cytometry for SH-SY5Y cells treated with or without 500nM rotenone for 16h (C). Dot plot showed annexin V-FITC in x-axis and PI in y-axis. Cells in the fourth quadrant undergoing early stage apoptosis are annexin V-positive/PI negative. And cells at late stage apoptosis or necrotic cells are both annexin V-FITC and PI positive. The left represents the untreated cells as the control. The apoptotic rates are shown as the average ratio ± SEM (n = 3).
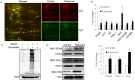
Fig 2. Analysis of S-nitrosoproteomes in SH-SY5Y cells with and without rotenone treatment using CyDye switch/2D-DIGE and BST/Western blot.
(A) The representative 2D-DIGE images. The internal standard (Cy3, green) and the target samples (Cy5, red) have been defined in the Methods section. The gel images were acquired by fluorescence scanning at λex = 548 nm and λem = 560 nm for Cy3-labelled samples and at λex = 641 nm and λem = 660 nm for Cy5-labelled samples. In the merged image, the circled spots represent the spots that differentially responded to rotenone treatment. (B) Comparison of the spot volumes for the seven differential proteins on 2D-DIGE. The spot fold-changes corresponding to the differential spots on Fig. 2A were analyzed using PDQuest software version 8.0.1. The differential spots were defined as changes in spot volume over 1.5-fold in all the cases, and were excised and tryptic digested for protein identification with mass spectrometry (fold-changes of spot volumes ± SEM, n = 3, *P<0.05 versus vehicle-treated group). (C) Specificity and efficiency of biotin switch technique. SH-SY5Y cell lysates were treated with or without 200 μM SNOC followed by BST. Protein extract was loaded onto an SDS-PAGE. Western blot analysis was carried out, and the membrane was probed with anti-biotin. Control samples were subjected to PBS or SNOC but not to ascorbate. (D) Verification of the proteins with increased S-nitrosylation in SH-SY5Y cells treated with rotenone treatment. The proteins were extracted from SH-SY5Y cells with and without rotenone treatment and subjected to BST, and the biotinylated proteins were pulled down. The potentially S-nitrosylated proteins were examined by Western blot using the corresponding antibodies. The fold-changes are shown in the bar chart (n = 3, *P<0.05 versus vehicle-treated group).
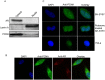
Fig 3. Localization of PCNA in SH-SY5Y cells.
(A) Localization of PCNA in the cytosolic and nuclear fractions of SH-SY5Y cells was examined by Western blot with antibodies against PCNA, Lamin A (nuclei marker), and aldose reductase (AR, cytosolic marker). (B) Localization of PCNA in SH-SY5Y cells was observed by confocal microscopy. Signal for aldose reductase served as cytosolic marker. (C) Effect to the localization of PCNA in SH-SY5Y cells by rotenone treatment was monitored by confocal microscopy with immunofluorescence using the PCNA antibody. DAPI was used as a nuclei stain.
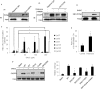
Fig 4. Effects of the S-nitrosylation status of PCNA on the interactions of PCNA and caspase-9.
(A) Diagonal CoIP using two antibodies against PCNA and caspase-9 in SH-SY5Y cells. The interactions of PCNA and caspase-9 were negatively correlated with the NO contents in SH-SY5Y cells. L-NMMA was used for the inhibition of nNOS, and rabbit IgG was used as a negative control for immunoprecipitation. (B) The S-nitrosylation of recombinant PCNA, identified by BST/Western blot, using SNOC as a NO donor. (C) Comparison of the sensitivity of the potential cysteine residues of recombinant PCNA to S-nitrosylation under different NO stress levels. The sensitivity of cysteine residues to NO modification is represented as the ratios of the S-nitrosylated peptides identified by LC-MS/MS to the sum of the corresponding peptides, which include all S-nitrosylated and non-S-nitrosylated peptides at certain sites (n = 3, *P<0.05). (D) Effects of the PCNA mutants under NO stress on the interactions of PCNA and caspase-9. In the pull-down experiment, the wild-type PCNA and three PCNA mutants, PCNA-C81A,-C162A and-C81A/C162A, were treated with SNOC and incubated with the HeLa cytosol, followed by enrichment with nickel-agarose beads and detection with Western blot using an antibody against caspase-9. The left panel shows the Western blot image, and the right panel presents the interaction of caspase-9 with different SNOC-modified recombinant PCNAs. The relative immune-recognition intensities were estimated based on the ratios of the specific band volume against the total band volumes for caspase-9 in the upper panel (n = 3, *P<0.05 versus WT PCNA). (E) Comparison of the S-nitrosylated status of PCNA at Cys81 in SH-SY5Y cells with and without rotenone treatment. The S-nitrosylation status of PCNA at Cys81 is represented as the ratios of the S-nitrosylated Cys81 peptide to the sum of the peptides that contained Cys81, which were identified by LC-MS/MS (n = 3, *P<0.05).
Fig 5. Regulation of caspase-9 activity by the S-nitrosylation status of PCNA.
(A) Recombinant wild-type PCNA was immobilized onto nickel-agarose beads followed by incubation with and without SNOC. After the incubated beads were added to the HeLa cytosolic extracts, the activity of caspase-9 cleavage was measured by Western blot using an anti-caspase-9 antibody. As a control, DTT was used to reduce the S-nitrosylation of PCNA induced by SNOC. The relative intensities of cleaved caspase-9 are shown in the bar chart (n = 3, *P<0.05 versus blank group). (B) Western blot analysis to PCNA overexpression and S-nitrosylation status in SH-SY5Y cells that were transfected with pcDNA3.1-PCNA. (C) Caspase-9 cleavage assay in SH-SY5Y cells with or without PCNA overexpression. The cells were treated with 500 nM rotenone for 16h followed by the cleavage activity assay based upon Western blot using antibody against caspase-9. (D) Annexin V-FITC/PI dual staining assay for rotenone-induced apoptosis in the SH-SY5Y cells which were WT, C81A, C162A, and C81/162A PCNA overexpressed, respectively. The apoptotic rates are shown as the average ratio ± SEM (n = 3).
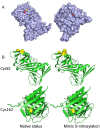
Fig 6. Potential structural consequences of S-nitrosylation for PCNA.
(A) The surface accessibility of S-nitrosylation sites. The sulfhydryl group (yellow) of Cys81 was exposed at the protein surface (left), but that of Cys162 was buried inside (right). (B) Molecular simulations of wild-type and SNOC-modified PCNAs. S-nitrosylation at Cys81 clearly affected the local structure of this residue, which may interfere with the interactions, but the Cys162 modification had no significant influence on the structure.
Similar articles
- S-Nitrosylation of OTUB1 Alters Its Stability and Ubc13 Binding.
Kumari R, Kumar R, Dey AK, Saha S, Maiti TK. Kumari R, et al. ACS Chem Neurosci. 2022 May 18;13(10):1517-1525. doi: 10.1021/acschemneuro.1c00855. Epub 2022 May 2. ACS Chem Neurosci. 2022. PMID: 35500217 - The neuroprotective role of ferrostatin-1 under rotenone-induced oxidative stress in dopaminergic neuroblastoma cells.
Kabiraj P, Valenzuela CA, Marin JE, Ramirez DA, Mendez L, Hwang MS, Varela-Ramirez A, Fenelon K, Narayan M, Skouta R. Kabiraj P, et al. Protein J. 2015 Oct;34(5):349-58. doi: 10.1007/s10930-015-9629-7. Protein J. 2015. PMID: 26385697 - Quantitative Proteomic Approaches for Analysis of Protein S-Nitrosylation.
Qu Z, Greenlief CM, Gu Z. Qu Z, et al. J Proteome Res. 2016 Jan 4;15(1):1-14. doi: 10.1021/acs.jproteome.5b00857. Epub 2015 Nov 23. J Proteome Res. 2016. PMID: 26544640 Review. - Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases.
Nakamura T, Lipton SA. Nakamura T, et al. Cell Death Differ. 2011 Sep;18(9):1478-86. doi: 10.1038/cdd.2011.65. Epub 2011 May 20. Cell Death Differ. 2011. PMID: 21597461 Free PMC article. Review.
Cited by
- Deciphering the Path of S-nitrosation of Human Thioredoxin: Evidence of an Internal NO Transfer and Implication for the Cellular Responses to NO.
Almeida VS, Miller LL, Delia JPG, Magalhães AV, Caruso IP, Iqbal A, Almeida FCL. Almeida VS, et al. Antioxidants (Basel). 2022 Jun 24;11(7):1236. doi: 10.3390/antiox11071236. Antioxidants (Basel). 2022. PMID: 35883729 Free PMC article. - Characterization of the Interaction Between SARS-CoV-2 Membrane Protein (M) and Proliferating Cell Nuclear Antigen (PCNA) as a Potential Therapeutic Target.
Zambalde ÉP, Pavan ICB, Mancini MCS, Severino MB, Scudero OB, Morelli AP, Amorim MR, Bispo-Dos-Santos K, Góis MM, Toledo-Teixeira DA, Parise PL, Mauad T, Dolhnikoff M, Saldiva PHN, Marques-Souza H, Proenca-Modena JL, Ventura AM, Simabuco FM. Zambalde ÉP, et al. Front Cell Infect Microbiol. 2022 May 23;12:849017. doi: 10.3389/fcimb.2022.849017. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35677658 Free PMC article. - Moesin is an effector of tau-induced actin overstabilization, cell cycle activation, and neurotoxicity in Alzheimer's disease.
Beckmann A, Ramirez P, Gamez M, Gonzalez E, De Mange J, Bieniek KF, Ray WJ, Frost B. Beckmann A, et al. iScience. 2023 Feb 8;26(3):106152. doi: 10.1016/j.isci.2023.106152. eCollection 2023 Mar 17. iScience. 2023. PMID: 36879821 Free PMC article. - Identification of new targets of S-nitrosylation in neural stem cells by thiol redox proteomics.
Santos AI, Lourenço AS, Simão S, Marques da Silva D, Santos DF, Onofre de Carvalho AP, Pereira AC, Izquierdo-Álvarez A, Ramos E, Morato E, Marina A, Martínez-Ruiz A, Araújo IM. Santos AI, et al. Redox Biol. 2020 May;32:101457. doi: 10.1016/j.redox.2020.101457. Epub 2020 Feb 7. Redox Biol. 2020. PMID: 32088623 Free PMC article. - Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress.
Akbar M, Essa MM, Daradkeh G, Abdelmegeed MA, Choi Y, Mahmood L, Song BJ. Akbar M, et al. Brain Res. 2016 Apr 15;1637:34-55. doi: 10.1016/j.brainres.2016.02.016. Epub 2016 Feb 13. Brain Res. 2016. PMID: 26883165 Free PMC article. Review.
References
- Michel PP, Ruberg M, Hirsch E (2006) Dopaminergic neurons reduced to silence by oxidative stress: an early step in the death cascade in Parkinson’s disease? Sci STKE 332: pe19. - PubMed
- Singh S, Dikshit M (2007) Apoptotic neuronal death in Parkinson’s disease: Involvement of nitric oxide. Brain Res Rev 54: 233–250. - PubMed
- Mochizuki H, Goto K, Mori H, Mizuno Y (1996) Histochemical detection of apoptosis in Parkinson’s disease. J Neurol Sci 137: 120–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
Funding was provided by National Key Basic Research Program of China: 2010CB912703; National High Technology Research and Development Program of China: 2012AA020205; Nature Science Foundation of China: 91131009. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous