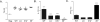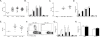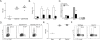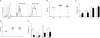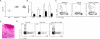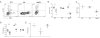Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation - PubMed (original) (raw)
. 2015 Feb 17;42(2):356-366.
doi: 10.1016/j.immuni.2015.01.008. Epub 2015 Feb 10.
Botond Z Igyarto # 1, Maryam Gerami-Nejad 2, Yosuke Kumamoto 3, Javed A Mohammed 1, Elizabeth Jarrett 1, Rebecca A Drummond 4, Sandra M Zurawski 5, Gerard Zurawski 5, Judith Berman 2 6, Akiko Iwasaki 3, Gordon D Brown 4, Daniel H Kaplan 1
Affiliations
- PMID: 25680275
- PMCID: PMC4343045
- DOI: 10.1016/j.immuni.2015.01.008
Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation
Sakeen W Kashem et al. Immunity. 2015.
Abstract
Candida albicans is a dimorphic fungus responsible for chronic mucocutaneous and systemic infections. Mucocutaneous immunity to C. albicans requires T helper 17 (Th17) cell differentiation that is thought to depend on recognition of filamentous C. albicans. Systemic immunity is considered T cell independent. Using a murine skin infection model, we compared T helper cell responses to yeast and filamentous C. albicans. We found that only yeast induced Th17 cell responses through a mechanism that required Dectin-1-mediated expression of interleukin-6 (IL-6) by Langerhans cells. Filamentous forms induced Th1 without Th17 cell responses due to the absence of Dectin-1 ligation. Notably, Th17 cell responses provided protection against cutaneous infection while Th1 cell responses provided protection against systemic infection. Thus, C. albicans morphology drives distinct T helper cell responses that provide tissue-specific protection. These findings provide insight into compartmentalization of Th cell responses and C. albicans pathogenesis and have critical implications for vaccine strategies.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
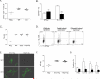
Figure 1. Candida albicans yeast drives Th17 cell differentiation in vivo
(A) The number of 2W1S-specific CD4+ T cells in six skin draining lymph nodes (axillary, brachial, inguinal) and spleen 8 days after mock (Neg) or skin infection with Eno1-Ag in wildtype (WT) or LC deficient mice (LC− mice is shown. (B) Percentage of PMA and Ionomycin stimulated 2W1S-specific cells in WT (white) or LC− (black) mice expressing the indicated cytokines. (C) Antigen was targeted to huLangerin transgenic mice by i.p injection of 1.0 μg 2G3-Eα. Adjuvant was provided by skin infection with wild-type, cph1/cph1, or tup1/tup1 strains of C. albicans. TEα numbers were assessed in the skin draining lymph nodes of mice 4 days after infection. Neg refers to unimmunized mice. (D) The expression of IFN-γ and IL-17A in PMA + Ionomycin stimulated Eα-specific CD4+ TEα cells from (C) is shown. (E) GFP expression in _Eno1_-Ag or _Hwp1_-Ag C. albicans under yeast or filamentous growth conditions was determined by immunofluorescence. (F) TEα expansion and (G) cytokines production 4 days after infection of WT mice with _Eno1_-Ag (white) or _Hwp1_-Ag (black) or naïve (Neg). Each symbol represents data from an individual animal. Scales represents mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.Data is representative of two to three independent experiments. See also Figure S1 and S2.
Figure 2. IL-1β and IL-6 are required for Th17 cell differentiation during C. albicans skin infection
(A) Expansion of TEα cells isolated from skin draining lymph nodes 4 days after skin infection of the indicated mouse strains with Eno1-Ag C. albicans. Each symbol represents results from an individual animal. (B) The percent of ex vivo PMA and Ionomycin stimulated TEα cells in cutaneous lymph nodes that express IL-17A or (C) IFN-γ as determined intracellular flow cytometry is shown. Neg refers to mock infected WT mice. Scales represents mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Data is representative of at least three independent experiments.
Figure 3. LC derived IL-6, but not TGF-β or IL-1β is required for Th17 cell differentiation
(A) huLangerin-creERT2 _Tgfb1_f/f mice were treated with tamoxifen to generate LC-specific ablation of TGF-β1. These mice were skin infected with Eno1-Ag C. albicans along with tamoxifen treated huLangerin-creERT2 _Tgfbr2_f//f and YFP controls. Expansion of TEα at day 4 along with (B) cytokines expression is shown. (C) Expansion and (D) cytokine production by TEα cells in Eno1-Ag infected WT→WT (white) or WT→_Il1b_−/− (black) bone marrow chimeric mice is shown. (E) The expansion, (F) expression of IL-17A by TEα cells and (G) percent of TEα cells producing the indicated cytokines in WT→WT (white) or WT→_Il6_−/− (black) mice is shown. (H) Cytokine production by TEα cells in WT→WT (white) or _Il6_−/− WT (black) mice is shown. Neg refers to mock infected WT mice. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. Data is representative of at least three independent experiments. See also Figure S3.
Figure 4. Dectin-1 engagement on LC leads to IL-6 production and Th17 cell differentiation
Antigen was directed to LC in huLangerin transgenic by i.p. injection of 1.0 μg 2G3-Eα followed by skin infection with SC5314 (white) or s20175.016 (black) strains of C. albicans. (A) TEα expansion and (B) cytokine production by TEα cells is shown. (C) LC were isolated from the skin draining lymph nodes by flow cytometry sorting on day 3 after skin infection with vehicle (white), SC5314 (gray) or s20175.016 (black). Expression of the indicated cytokines was determined by qPCR and is shown normalized to uninfected mice. (D) Antigen was targeted to LC (as in (A)) followed by infection with SC5314 or S20175.016. At the time of infection, PBS or 1.0 μg of rIL-6 was injected at the site of infection. CSFE dilution of TEα cells as well as expression of IL-17A is shown. (E) Expansion of TEα cells in the skin draining lymph nodes of WT and Dectin-1 deficient mice 4 days after infection with _Eno1_-Ag. (F) CSFE dilution and expression of IL-17A in PMA and Ionomycin stimulated TEα cells isolated from WT and Dectin-1 deficient mice on day 4 after infection. Neg refers to mock infected WT mice. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. Data is representative of at least two independent experiments. See also Figure S4 and S5
Figure 5. CD11b+ Dermal DC are not required for Th17 cell differentiation
(A) Surface expression of Dectin-1 by the indicated skin DC subset isolated from the skin under steady-state conditions is shown. (B) Mgl2-DTR→WT chimeric mice were treated with vehicle (white circles) or diphtheria toxin (black circles) and skin infected with Eno1-Ag 1 day later. The expansion of TEα cells isolated from skin draining lymph nodes 4 days after infection is shown. “Neg” represents mock infected WT mice (triangles). (C) Expression of the indicated cytokines from PMA and Ionomycin TEa cells in (B) is shown. (D) Expansion and (E) cytokine production by TEα cells in WT (white) or LC−x_Batf3_−/− (black) mice 4 days after infection with Eno1-Ag is shown. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. Data is representative of at least three independent experiments. See also Figure S6.
Figure 6. Dermal infection and C. albicans hyphae do not induce Th17 cells without exogenous Dectin-1 ligands
(A and B) WT mice were infected with an equivalent inoculum of _Eno1_-Ag C. albicans on the epidermis (white) or by intradermal injection (black). Expansion and cytokine production by PMA and Ionomycin stimulated TEα cells isolated from skin draining lymph nodes is shown. (C) As in (A), WT mice were infected epicutaneously or by dermal injection into which purified curdlan or vehicle has been added to the inoculum. CSFE dilution and expression of IL-17A by PMA and Ionomycin stimulated TEα cells is shown. (D) PAS stain of skin 2 days after C. albicans infection showing budding yeast (asterisk) restricted to the epidermis and numerous penetrating hyphae in the dermis (arrowhead) (bar=150uM). (E) The expression of IL-17A and IFNγ by PMA and Ionomycin stimulated TEα cells cells 4 days after epicutaneous infection of WT mice with Eno1-Ag or Hwp1-Ag is shown. Curdlan or vehicle alone was provided by dermal injection at the time of infection. “Neg” represents mock infected WT mice. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. Data is representative of at least three independent experiments. See also Figure S7.
Figure 7. Th1 and Th17 cells provide compartmentalized protection to C. albicans
WT, _Il6_−/− and _Batf3_−/− mice were epicutaneously infected with _Eno1_-Ag. (A) The expression of IL-17A and IFN-γ by PMA and Ionomycin stimulated CD4+ TEα cells isolated from skin draining lymph nodes 4 days after infection is shown. (B) The indicated strains of mice were mock infected (white) or skin infected with SC5314. Mice were re-challenged in the skin with SC5314 9 days after spontaneous clearance of the initial infection. Fungal skin burdens of individual mice is shown as CFU/cm2. (C) CD4+ T cells were purified from skin draining LN and spleen of naïve WT, SC5314 infected _Il6_−/− or SC5314 infected _Batf_3−/− mice 7 days after infection and adoptively transferred into into naïve WT recipients. Recipients were skin infected with SC5314 1 day after transfer and CFU was assessed at the infected area 2 days later. (D) As in (B) except that mice were challenged by i.v. infection with 105 SC5314. (E) As in (C) except that mice were challenged by i.v. infection with 105 SC5314. Fungal burdens in the kidneys expressed as CFU/g is shown. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001.
Comment in
- Mushrooming insights into skin dendritic cell physiology.
Nagao K, Udey MC. Nagao K, et al. Immunity. 2015 Feb 17;42(2):210-213. doi: 10.1016/j.immuni.2015.01.025. Immunity. 2015. PMID: 25692697
Similar articles
- Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses.
Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Igyártó BZ, et al. Immunity. 2011 Aug 26;35(2):260-72. doi: 10.1016/j.immuni.2011.06.005. Epub 2011 Jul 21. Immunity. 2011. PMID: 21782478 Free PMC article. - Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo.
d'Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. d'Ostiani CF, et al. J Exp Med. 2000 May 15;191(10):1661-74. doi: 10.1084/jem.191.10.1661. J Exp Med. 2000. PMID: 10811860 Free PMC article. - Mushrooming insights into skin dendritic cell physiology.
Nagao K, Udey MC. Nagao K, et al. Immunity. 2015 Feb 17;42(2):210-213. doi: 10.1016/j.immuni.2015.01.025. Immunity. 2015. PMID: 25692697 - Adaptive immune responses to Candida albicans infection.
Richardson JP, Moyes DL. Richardson JP, et al. Virulence. 2015;6(4):327-37. doi: 10.1080/21505594.2015.1004977. Epub 2015 Jan 21. Virulence. 2015. PMID: 25607781 Free PMC article. Review. - Initiation of T-helper cell immunity to Candida albicans by IL-12: the role of neutrophils.
Romani L, Bistoni F, Puccetti P. Romani L, et al. Chem Immunol. 1997;68:110-35. doi: 10.1159/000058688. Chem Immunol. 1997. PMID: 9329219 Review.
Cited by
- Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion.
Qin Z, Bouteau A, Herbst C, Igyártó BZ. Qin Z, et al. bioRxiv [Preprint]. 2022 Aug 20:2022.03.16.484616. doi: 10.1101/2022.03.16.484616. bioRxiv. 2022. PMID: 36032972 Free PMC article. Updated. Preprint. - Multi-Omics Profiling of Candida albicans Grown on Solid Versus Liquid Media.
Alhameed RA, Semreen MH, Hamad M, Giddey AD, Sulaiman A, Al Bataineh MT, Al-Hroub HM, Bustanji Y, Alzoubi KH, Soares NC. Alhameed RA, et al. Microorganisms. 2023 Nov 22;11(12):2831. doi: 10.3390/microorganisms11122831. Microorganisms. 2023. PMID: 38137975 Free PMC article. - They shall not grow mold: Soldiers of innate and adaptive immunity to fungi.
Woodring T, Deepe GS, Levitz SM, Wuethrich M, Klein BS. Woodring T, et al. Semin Immunol. 2023 Jan;65:101673. doi: 10.1016/j.smim.2022.101673. Epub 2022 Nov 29. Semin Immunol. 2023. PMID: 36459927 Free PMC article. Review. - Dectin-1 Exerts Dual Control in the Gut.
Iliev ID. Iliev ID. Cell Host Microbe. 2015 Aug 12;18(2):139-41. doi: 10.1016/j.chom.2015.07.010. Cell Host Microbe. 2015. PMID: 26269949 Free PMC article. - Pathogen size alters C-type lectin receptor signaling in dendritic cells to influence CD4 Th9 cell differentiation.
Oh S, Li K, Prince A, Wheeler ML, Hamade H, Nguyen C, Michelsen KS, Underhill DM. Oh S, et al. Cell Rep. 2022 Mar 29;38(13):110567. doi: 10.1016/j.celrep.2022.110567. Cell Rep. 2022. PMID: 35354044 Free PMC article.
References
- Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend ARM, Drakesmith H. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129–4139. - PubMed
- Bär E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 Regulates Systemic Fungal Immunity by Controlling the Functional Competence of NK Cells. Immunity. 2014 - PubMed
- Bonifazi P, Zelante T, D'Angelo C, De Luca A, Moretti S, Bozza S, Perruccio K, Iannitti RG, Giovannini G, Volpi C, et al. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2:362–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR060744/AR/NIAMS NIH HHS/United States
- R01 AI054359/AI/NIAID NIH HHS/United States
- R01 AR056632/AR/NIAMS NIH HHS/United States
- AR056632/AR/NIAMS NIH HHS/United States
- AR060744/AR/NIAMS NIH HHS/United States
- 102705/WT_/Wellcome Trust/United Kingdom
- T32 AI007313/AI/NIAID NIH HHS/United States
- P30 CA077598/CA/NCI NIH HHS/United States
- T32 GM008244/GM/NIGMS NIH HHS/United States
- 102705/Z/13/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases