Microtubule motors power plasma membrane tubulation in clathrin-independent endocytosis - PubMed (original) (raw)
doi: 10.1111/tra.12269. Epub 2015 Apr 27.
Nicholas W Baetz 1, Courtney A Copeland 1, Lewis J Kraft 3, Bing Han 1, Ajit Tiwari 1, Kimberly R Drake 1, Heidi De Luca 4, Daniel J-F Chinnapen 4, Michael W Davidson 5, Randall K Holmes 6, Michael G Jobling 6, Trina A Schroer 7, Wayne I Lencer 4 8, Anne K Kenworthy 1 3 9 10
Affiliations
- PMID: 25690058
- PMCID: PMC4440230
- DOI: 10.1111/tra.12269
Microtubule motors power plasma membrane tubulation in clathrin-independent endocytosis
Charles A Day et al. Traffic. 2015 Jun.
Abstract
How the plasma membrane is bent to accommodate clathrin-independent endocytosis remains uncertain. Recent studies suggest Shiga and cholera toxin induce membrane curvature required for their uptake into clathrin-independent carriers by binding and cross-linking multiple copies of their glycosphingolipid receptors on the plasma membrane. But it remains unclear if toxin-induced sphingolipid crosslinking provides sufficient mechanical force for deforming the plasma membrane, or if host cell factors also contribute to this process. To test this, we imaged the uptake of cholera toxin B-subunit into surface-derived tubular invaginations. We found that cholera toxin mutants that bind to only one glycosphingolipid receptor accumulated in tubules, and that toxin binding was entirely dispensable for membrane tubulations to form. Unexpectedly, the driving force for tubule extension was supplied by the combination of microtubules, dynein and dynactin, thus defining a novel mechanism for generating membrane curvature during clathrin-independent endocytosis.
Keywords: cholera toxin; clathrin-independent endocytosis; dynactin; dynein; membrane curvature; microtubules.
© 2015 The Authors. Traffic published by John Wiley & Sons Ltd.
Figures
Figure 1
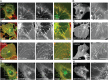
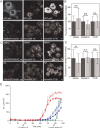
No more than one functional GM1 binding site is required to target cholera toxin to plasma membrane invaginations. A–E) CTxB accumulates in either linear extended tubules (A–D) or branched tubules (E) under conditions that block scission. Bar, 10 µm. (F–M) Cholera toxin binding mutants accumulate in tubular invaginations. F) Cy3-CTx chimera labels tubules in ATP depleted COS-7 cells. G–I) Quantification of invaginations in ATP-depleted cells labeled with Cy3-CTx chimera or Alexa555-CTxB. G) Percentage of cells displaying invaginations (mean ± SD from 117–119 cells). *, p < 0.05, chi-squared test. H) Average number of invaginations per cell (mean ± SD of 42–46 cells). _n.s._, _p_ > 0.05; Student _t_-test. I) Length of invaginations (mean ± SD for 219–332 invaginations). n.s., p > 0.05; Student _t_-test. J–M) Both wild type CTxB and monovalent CTx accumulate in tubular invaginations in cells subjected to Jasplakinolide pretreatment prior to ATP depletion. L) Percentage of cells displaying invaginations. (mean ± SD of 59–63 cells). n.s., p > 0.05; chi-squared test. M) Average number of invaginations per cell. (mean ± SD of 59–63 cells). n.s., p > 0.05; Student _t_-test. N and O) Similar to wild type CTxB, monovalent CTx accumulates in branched tubules in Dynasore-treated cells. Bars, 10 µm.
Figure 2
Toxin binding is not necessary for tubular invaginations to form. A,B) EGFP-HRas (green) is found in plasma membrane invaginations in ATP-depleted cells in both the presence (A) and absence (B) of Alexa555-CTxB (red). C–F) Similar results were obtained for GFP-HRas in cells subjected to actin disruption (C and D) or actin stabilization (E and F). G and H) A construct containing only the C-terminal 10 amino acids of HRas, EGFP-HRas-tail (green), also localized to tubules in both the presence and absence of CTxB. Bars, 10 µm.
Figure 3
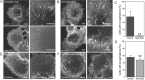
Tubular invaginations align along microtubules and undergo complex motions including bidirectional motility and branching events. A) Microtubules persist in RFP-α-tubulin expressing HeLa cells following ATP depletion. B) CTxB positive invaginations (green) align with taxol-stabilized microtubules (red) in stably expressing RFP-α-tubulin HeLa cells under ATP depletion. C) Percentage of CTxB-positive tubules that align with microtubules (black), partially align with microtubules (gray), or do not colocalize with microtubules (light gray) in ATP-depleted HeLa cells expressing RFP-α-tubulin. N = 84 cells. D–J) CTxB-enriched tubules exhibit complex motions in live cells as illustrated by representative frames from time series and corresponding kymographs. Time stamps are in minutes:seconds. D–F) CTxB-positive invaginations often grow in fluid directed motions. G–I) CTxB-positive invaginations also extend, retract and regrow along the same axis. J) Occasionally, the CTxB positive tubules undergo branching events. Bars, 10 µm.
Figure 4
An intact microtubule network is required for the formation of tubular invaginations. A and C) Microtubule disruption with high dose nocodazole prevents the formation of tubular invaginations in ATP-depleted cells (mean ± SD, N = 74 cells.) **p < 0.01, chi-squared test. B and D) Inhibition of microtubule dynamics with low dose nocodazole has no effect on the formation of tubular invaginations in ATP-depleted cells (mean ± SD, _N_ = 135–169 cells.) _n.s. p_ > 0.05, chi-squared test. E and F) Microtubule disruption prior to Dynasore treatment blocks the formation of branched tubules in cells labeled with either wild type CTxB (E) or monovalent CTx (F). Bars, 10 µm.
Figure 5
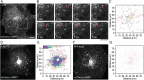
Motor-based motions persist in ATP-depleted cells. A–C) A subset of lysosomes labeled with mCherry-LAMP1 display long range directed motions in ATP-depleted cells. B) Time lapse of zoomed in region of the cell in panel A. An example of a lysosome undergoing long-range directed motion is marked with the arrowhead. C) Tracings of lysosome movement in the cell shown in A. Each track is indicated by a different color. D and E) Frequent long-range directed motions are observed under control conditions. F and G) mCherry-LAMP1 positive lysosomes are immobile in PFA fixed cells. Elapsed time in F, H and J = 470 s. Bars, 10 µm.
Figure 6
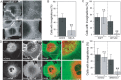
The ATPase activity of dynein and an intact dynactin complex are required for the formation of tubular invaginations. A and B) Inhibition of dynein ATPase activity with ciliobrevin-A (Cilio-A) significantly reduces the percent of cells displaying tubular invaginations (mean ± SD from 95–122 cells). **p < 0.01, chi-squared test. C) Expression of GFP-p50 reduced the prevalence of cells with invaginations as compared to untransfected cells (−) or cells expressing EGFP. (mean ± SD from 42–101 cells). _n.s_., _p_ > 0.05; **p < 0.01, chi-squared test. D and E) Expression of CC1-dsRed (red) significantly reduces the percent of cells with CTxB positive invaginations (green) as compared to untransfected cells (−) or control cells expressing mCherry (red). (mean ± SD from 40–64 cells). _n.s._, _p_ > 0.05; **p < 0.01, chi-squared test. Bars, 10 µm.
Figure 7
Bulk endocytosis of CTxB is largely unaffected by disruption of microtubules or the dynactin complex. A and B) Endocytosis of dextran, transferrin and CTxB in cells expressing GFP-p50 (gray bars) compared to cells expressing GFP (black bars). B shows mean ± SD for 37–278 cells. n.s., p > 0.05, Student's _t_-test. C and D) Effect of microtubule disruption with 5 µg/mL nocodazole (gray bars) on the uptake of dextran, transferrin and CTxB. Control cells were treated with DMSO (black bars). D shows mean ± SD for 78–358 cells. n.s., p > 0.05, *, p < 0.05, Student's _t_-test. Bars, 10 µm. E) Representative time course of toxin-induced chloride secretion in T84 cells in response to treatment with 20 nM wt CTx. Cells were either pretreated with NZ (closed symbols) or with DMSO (open symbols) prior to toxin addition to either the apical (blue) or basolateral (red) surface at t = 30 min as described in the Materials and Methods. Forskolin was added to control monolayers (green or gray diamonds) at 90 min in order to demonstrate equivalency of the secretory response and monolayer viability. The error bars indicate the variance calculated as the standard deviation (n = 3). Data are representative of the results of two independent experiments.
Figure 8
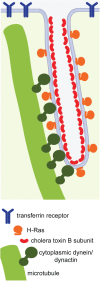
Working model: microtubules, dynactin and cytoplasmic dynein facilitate plasma membrane tubulation. Dynein and dynactin provide attachment sites or generate tugging forces on the plasma membrane, leading to microtubule-dependent tubulation. Glycolipid-binding toxins may further sense, induce or stabilize membrane curvature, enabling their efficient sorting into tubular structures.
Similar articles
- Structured clustering of the glycosphingolipid GM1 is required for membrane curvature induced by cholera toxin.
Kabbani AM, Raghunathan K, Lencer WI, Kenworthy AK, Kelly CV. Kabbani AM, et al. Proc Natl Acad Sci U S A. 2020 Jun 30;117(26):14978-14986. doi: 10.1073/pnas.2001119117. Epub 2020 Jun 17. Proc Natl Acad Sci U S A. 2020. PMID: 32554490 Free PMC article. - Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells.
Glebov OO, Bright NA, Nichols BJ. Glebov OO, et al. Nat Cell Biol. 2006 Jan;8(1):46-54. doi: 10.1038/ncb1342. Epub 2005 Dec 11. Nat Cell Biol. 2006. PMID: 16341206 - Shiga toxin stimulates clathrin-independent endocytosis of the VAMP2, VAMP3 and VAMP8 SNARE proteins.
Renard HF, Garcia-Castillo MD, Chambon V, Lamaze C, Johannes L. Renard HF, et al. J Cell Sci. 2015 Aug 1;128(15):2891-902. doi: 10.1242/jcs.171116. Epub 2015 Jun 12. J Cell Sci. 2015. PMID: 26071526 - Cholera Toxin as a Probe for Membrane Biology.
Kenworthy AK, Schmieder SS, Raghunathan K, Tiwari A, Wang T, Kelly CV, Lencer WI. Kenworthy AK, et al. Toxins (Basel). 2021 Aug 3;13(8):543. doi: 10.3390/toxins13080543. Toxins (Basel). 2021. PMID: 34437414 Free PMC article. Review. - Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling.
Donaldson JG, Porat-Shliom N, Cohen LA. Donaldson JG, et al. Cell Signal. 2009 Jan;21(1):1-6. doi: 10.1016/j.cellsig.2008.06.020. Epub 2008 Jul 3. Cell Signal. 2009. PMID: 18647649 Free PMC article. Review.
Cited by
- Actin modulates shape and mechanics of tubular membranes.
Allard A, Bouzid M, Betz T, Simon C, Abou-Ghali M, Lemière J, Valentino F, Manzi J, Brochard-Wyart F, Guevorkian K, Plastino J, Lenz M, Campillo C, Sykes C. Allard A, et al. Sci Adv. 2020 Apr 22;6(17):eaaz3050. doi: 10.1126/sciadv.aaz3050. eCollection 2020 Apr. Sci Adv. 2020. PMID: 32494637 Free PMC article. - Physical basis of some membrane shaping mechanisms.
Simunovic M, Prévost C, Callan-Jones A, Bassereau P. Simunovic M, et al. Philos Trans A Math Phys Eng Sci. 2016 Jul 28;374(2072):20160034. doi: 10.1098/rsta.2016.0034. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27298443 Free PMC article. Review. - CRACR2a is a calcium-activated dynein adaptor protein that regulates endocytic traffic.
Wang Y, Huynh W, Skokan TD, Lu W, Weiss A, Vale RD. Wang Y, et al. J Cell Biol. 2019 May 6;218(5):1619-1633. doi: 10.1083/jcb.201806097. Epub 2019 Feb 27. J Cell Biol. 2019. PMID: 30814157 Free PMC article. - Glycolipid Crosslinking Is Required for Cholera Toxin to Partition Into and Stabilize Ordered Domains.
Raghunathan K, Wong TH, Chinnapen DJ, Lencer WI, Jobling MG, Kenworthy AK. Raghunathan K, et al. Biophys J. 2016 Dec 20;111(12):2547-2550. doi: 10.1016/j.bpj.2016.11.008. Epub 2016 Nov 30. Biophys J. 2016. PMID: 27914621 Free PMC article. - Membrane curvature in cell biology: An integration of molecular mechanisms.
Jarsch IK, Daste F, Gallop JL. Jarsch IK, et al. J Cell Biol. 2016 Aug 15;214(4):375-87. doi: 10.1083/jcb.201604003. J Cell Biol. 2016. PMID: 27528656 Free PMC article. Review.
References
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. - PubMed
- Sandvig K, Pust S, Skotland T, van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Curr Opin Cell Biol. 2011;23:413–420. - PubMed
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. - PubMed
- Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr Opin Cell Biol. 2010;22:519–527. - PubMed
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK084424/DK/NIDDK NIH HHS/United States
- R01 GM44589/GM/NIGMS NIH HHS/United States
- R01 GM106720/GM/NIGMS NIH HHS/United States
- R44 EB008589/EB/NIBIB NIH HHS/United States
- DK48106/DK/NIDDK NIH HHS/United States
- R25 GM062459/GM/NIGMS NIH HHS/United States
- R21 DK090603/DK/NIDDK NIH HHS/United States
- T32 DK007013/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 DK34854/DK/NIDDK NIH HHS/United States
- T32 GM08320/GM/NIGMS NIH HHS/United States
- R44-EB008589/EB/NIBIB NIH HHS/United States
- R01 HL111259/HL/NHLBI NIH HHS/United States
- R01 AI031940/AI/NIAID NIH HHS/United States
- R01 AI31940/AI/NIAID NIH HHS/United States
- DK084424/DK/NIDDK NIH HHS/United States
- R01 GM044589/GM/NIGMS NIH HHS/United States
- T32 GM008320/GM/NIGMS NIH HHS/United States
- DK090603/DK/NIDDK NIH HHS/United States
- R01 DK048106/DK/NIDDK NIH HHS/United States
- DK020593/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- R01 GM073846/GM/NIGMS NIH HHS/United States
- R37 DK048106/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources