HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis - PubMed (original) (raw)
. 2015 Mar 3;112(9):2853-8.
doi: 10.1073/pnas.1501441112. Epub 2015 Feb 17.
Yejie Shi 2, Xiaoyan Jiang 3, Rehana K Leak 4, Xiaoming Hu 2, Yun Wu 2, Hongjian Pu 1, Wei-Wei Li 3, Bo Tang 3, Yun Wang 3, Yanqin Gao 1, Ping Zheng 3, Michael V L Bennett 5, Jun Chen 6
Affiliations
- PMID: 25691750
- PMCID: PMC4352818
- DOI: 10.1073/pnas.1501441112
HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis
Guohua Wang et al. Proc Natl Acad Sci U S A. 2015.
Abstract
Severe traumatic brain injury (TBI) elicits destruction of both gray and white matter, which is exacerbated by secondary proinflammatory responses. Although white matter injury (WMI) is strongly correlated with poor neurological status, the maintenance of white matter integrity is poorly understood, and no current therapies protect both gray and white matter. One candidate approach that may fulfill this role is inhibition of class I/II histone deacetylases (HDACs). Here we demonstrate that the HDAC inhibitor Scriptaid protects white matter up to 35 d after TBI, as shown by reductions in abnormally dephosphorylated neurofilament protein, increases in myelin basic protein, anatomic preservation of myelinated axons, and improved nerve conduction. Furthermore, Scriptaid shifted microglia/macrophage polarization toward the protective M2 phenotype and mitigated inflammation. In primary cocultures of microglia and oligodendrocytes, Scriptaid increased expression of microglial glycogen synthase kinase 3 beta (GSK3β), which phosphorylated and inactivated phosphatase and tensin homologue (PTEN), thereby enhancing phosphatidylinositide 3-kinases (PI3K)/Akt signaling and polarizing microglia toward M2. The increase in GSK3β in microglia and their phenotypic switch to M2 was associated with increased preservation of neighboring oligodendrocytes. These findings are consistent with recent findings that microglial phenotypic switching modulates white matter repair and axonal remyelination and highlight a previously unexplored role for HDAC activity in this process. Furthermore, the functions of GSK3β may be more subtle than previously thought, in that GSK3β can modulate microglial functions via the PTEN/PI3K/Akt signaling pathway and preserve white matter homeostasis. Thus, inhibition of HDACs in microglia is a potential future therapy in TBI and other neurological conditions with white matter destruction.
Keywords: inflammation; microglial polarization; myelination; oligodendrocyte; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
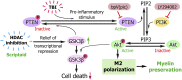
Fig. 1.
Proposed mechanism underlying the preservation of white matter by HDAC inhibition. Microglia/macrophages respond to TBI with proinflammatory changes that increase active, dephosphorylated PTEN. Active PTEN then converts phosphatidylinositol (3,5)-triphosphate (PIP3) to phosphatidyl (4,5)-biphosphate (PIP2), thereby inactivating the prosurvival kinase Akt and leading to microglial and oligodendroglial toxicity. HDAC inhibition counterbalances this inflammatory effect by increasing GSK3β, perhaps by relieving transcriptional repression of its mRNA. Active GSK3β then phosphorylates PTEN at Thr366, leading to a loss of PTEN activity. This loss of PTEN function results in activation of Akt, because PIP3 is no longer converted to PIP2. Activated Akt can now shift microglia from the destructive M1 phenotype toward the beneficial M2 phenotype and thereby elicit the protection of neighboring oligodendrocytes. Activated Akt also phosphorylates GSK3β, thereby disabling its pro-death effect. Knockdown of GSK3β or the PI3K/Akt inhibitor LY294002 each blocks the protective effects of HDAC inhibition, whereas the PTEN inhibitor bpV(pic) fails to provide any additional effect over that of HDAC inhibition.
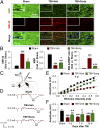
Fig. 2.
Long-term preservation of white matter after TBI is promoted by Scriptaid. (A) Double immunofluorescent staining for dephosphorylated neurofilament protein (SMI-32) and MBP in the ipsilesional CC at 35 d after TBI or sham treatment. Nuclei are labeled with DAPI (blue). (Insets) High-power images. (B) Degree of WMI, expressed as the fold increases of SMI-32, fold decreases of MBP, and the ratio of SMI-32 to MBP. (C) Illustration of the CCI lesion (dashed line) and regions for immunohistochemistry and electron microscropy (red box). Stimulating and recording electrodes were positioned at the CC as shown to measure the evoked CAPs. (D) Representative traces of the evoked CAPs in the CC (stimulus, 0.3 mA; 0.48 mm lateral to the stimulating electrode) at 7 d post-TBI. (E) Signal conduction along nerve fibers, as measured by the amplitude of the N1 component of the CAPs in response to increasing stimulus strength (0.05–0.55 mA) at 7 d post-TBI. (F) N1 amplitude in response to a 0.3-mA stimulus at 3, 7, and 35 d post-TBI. Shown are the mean ± SEM values from six mice per group. #P ≤ 0.05; ##P ≤ 0.01, ###P ≤ 0.001 vs. sham; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. TBI + vehicle.
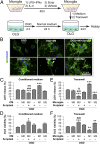
Fig. 3.
HDAC inhibition indirectly reduces oligodendrocyte injury by modulating microglia/macrophages. (A) In vitro experiments using a CM transfer system or a Transwell system. Microglia cultures (M0) were incubated with Scriptaid (1 μM) or vehicle for 48 h; as positive controls, microglia were primed toward M1 or M2 using LPS (100 ng/mL) plus IFN-γ (20 ng/mL) or IL-4 (20 ng/mL), respectively, for 48 h (18). Cultured oligodendrocytes were exposed to 2-h OGD or control, non-OGD conditions and returned to normal medium. Twenty-four hours later, microglia CM was applied to oligodendrocytes for 24 h by pipetting or by a Transwell system. OLG, oligodendrocytes. (B) MBP and DAPI staining of oligodendrocytes exposed to OGD and cultured with CM from microglia exhibiting M0 (with or without Scriptaid), M1, or M2 phenotypes. (C–F) Oligodendrocyte survival and cell death were quantified by LDH release and MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay, respectively, after exposure to CM (C and D) or in Transwells (E and F). M0 phenotype in conjunction with Scriptaid or the M2 phenotype protected oligodendrocyte viability after OGD, whereas the M1 phenotype decreased oligodendrocyte viability. Shown are the mean ± SEM values from three independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. OGD + OLG; ###P ≤ 0.001 vs. OGD + OLG + M0 microglia without Scriptaid; P ≤ 0.01, $P ≤ 0.001 vs. OGD + OLG + Scriptaid.
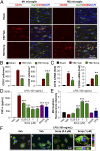
Fig. 4.
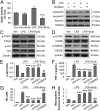
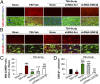
HDAC inhibition primes microglia toward the M2 phenotype in vitro and in vivo. (A) Double immunofluorescent staining for M1 marker CD16 or M2 marker CD206 with Iba1 marker for activated microglia in the ipsilesional CC at 7 d following TBI (vehicle and Scriptaid groups) or sham surgery (sham group). Shown is immunofluorescent staining for CD16 or CD206 (first or third column) and colabeling of the same sections for Iba1 and nuclei (DAPI; second and fourth columns). (B) Blinded cell counts of microglia immunolabeled for CD16 and CD206 from the same animals as shown in A. (C) Messenger RNA levels of CD16 and CD206 in the ipsilateral striatum at 7 d after TBI with or without Scriptaid. Scriptaid was injected at 3.5 mg/kg at 2 h after CCI, and this was repeated daily for the next 2 d (at 26 and 50 h after injury). (D) HDAC inhibitors Scriptaid, SAHA (2.5 µM), and VPA (4 μM) reduced LPS-induced production of TNF-α by microglia. (E) Microglial cultures were treated with LPS alone or in combination with Scriptaid, SAHA, or VPA for 48 h. Fluorescent microspheres were added for 3 h, and intramicroglial fluorescence intensity was measured. Scriptaid was more potent than SAHA or VPA in enhancing microsphere uptake by microglia. (F) Microglia were treated with LPS with or without Scriptaid as above and stained with phalloidin (green) to visualize F-actin. After a 3-h incubation, phagocytosed microspheres appeared red, and DAPI-stained nuclei appeared blue. Shown are the mean ± SEM values from four independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. LPS + vehicle.
Fig. 5.
HDAC inhibition modulates microglial polarization through the PI3K/Akt pathway. (A) LPS-induced HDAC activity in primary microglia cultures was reduced by Scriptaid. (B) Scriptaid prevented decreases in acetylated-histone 3 (H3) and acetylated H4 in primary microglia at 3 h after LPS, as measured by Western blot analysis. (C) Scriptaid prevented loss of p-PTEN (Ser380/Thr382/383) and p-Akt (Ser473) in LPS-challenged microglia, as measured by Western blot analysis. (D) Scriptaid largely prevented the decrease in p-mTOR (Ser2488) and p-GSK3β (Ser9) levels in LPS-treated microglia. (E) LPS reduced p-Akt levels, an effect largely blocked by the PTEN inhibitor dipotassium bisperoxo (picolinato) oxovanadate [bpV(pic)] (1 μM). Scriptaid partially preserved p-Akt levels in LPS-treated microglia alone or in the presence of bpV(pic), but not in the presence of the PI3K/Akt inhibitor LY294002 (10 µM). (F and G) LPS treatment of microglia increased TNF-α and NO production, an effect reduced by bpV(pic) and by Scriptaid. The effect or Scriptaid was reduced by LY294002, but not by bpV(pic). (H) Microsphere uptake was reduced by LPS alone, but increased by LPS + bpV(pic) or LPS + Scriptaid +/− bpV(pic); the effect of Scriptaid on LPS was blocked by LY294002. Thus, all LPS effects were opposed by Scriptaid, and the effects of Scriptaid were abolished by LY294002 and unchanged by [bpV(pic)]. Data are mean ± SEM values from four independent experiments. ***P ≤ 0.001 vs. LPS alone; ##P ≤ 0.01, ###P ≤ 0.001 vs. LPS + Scriptaid.
Fig. 6.
In vivo GSK3β knockdown attenuates Scriptaid-afforded protection against WMI. (A and C) Immunofluorescent staining for SMI-32 (upper row in A) and colabeling of the same sections for MBP and nuclei (DAPI) in the second row. Images were taken from the CC of animals at 7 d post-TBI. Lentiviral shRNA for GSK3β (shRNA-GSK3β) or scrambled shRNA (shRNA-Scr) was infused into the CC 7 d before TBI; Scriptaid was injected at 2, 26, and 50 h after TBI. WMI was expressed as the ratio of SMI-32 to MBP in the CC normalized to sham (C). (B and D) Immunofluorescent staining for GSK3β and Iba-1 in the CC at 7 d post-TBI as in A and C. Quantification of GSK3β+ cells is illustrated in D. Shown are the mean ± SEM values from six mice per group. #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 vs. sham; ***P ≤ 0.001 vs. vehicle; $P < 0.001 vs. Scriptaid.
Similar articles
- HDAC inhibition reduces white matter injury after intracerebral hemorrhage.
Yang H, Ni W, Wei P, Li S, Gao X, Su J, Jiang H, Lei Y, Zhou L, Gu Y. Yang H, et al. J Cereb Blood Flow Metab. 2021 May;41(5):958-974. doi: 10.1177/0271678X20942613. Epub 2020 Jul 23. J Cereb Blood Flow Metab. 2021. PMID: 32703113 Free PMC article. - Selective activation of cannabinoid receptor-2 reduces white matter injury via PERK signaling in a rat model of traumatic brain injury.
Li L, Luo Q, Shang B, Yang X, Zhang Y, Pan Q, Wu N, Tang W, Du D, Sun X, Jiang L. Li L, et al. Exp Neurol. 2022 Jan;347:113899. doi: 10.1016/j.expneurol.2021.113899. Epub 2021 Oct 20. Exp Neurol. 2022. PMID: 34678230 - Tetrahydrocurcumin Protects Against GSK3β/PTEN/PI3K/Akt-Mediated Neuroinflammatory Responses and Microglial Polarization Following Traumatic Brain Injury.
Zhang J, Gu Y, Sun W, Yu L, Li T. Zhang J, et al. Mol Neurobiol. 2024 Sep;61(9):7026-7036. doi: 10.1007/s12035-024-04034-6. Epub 2024 Feb 17. Mol Neurobiol. 2024. PMID: 38368289 - Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury.
Armstrong RC, Mierzwa AJ, Sullivan GM, Sanchez MA. Armstrong RC, et al. Neuropharmacology. 2016 Nov;110(Pt B):654-659. doi: 10.1016/j.neuropharm.2015.04.029. Epub 2015 May 9. Neuropharmacology. 2016. PMID: 25963414 Review. - White matter involvement after TBI: Clues to axon and myelin repair capacity.
Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. Armstrong RC, et al. Exp Neurol. 2016 Jan;275 Pt 3:328-333. doi: 10.1016/j.expneurol.2015.02.011. Epub 2015 Feb 16. Exp Neurol. 2016. PMID: 25697845 Review.
Cited by
- The dual function of microglial polarization and its treatment targets in ischemic stroke.
Mo Y, Xu W, Fu K, Chen H, Wen J, Huang Q, Guo F, Mo L, Yan J. Mo Y, et al. Front Neurol. 2022 Sep 23;13:921705. doi: 10.3389/fneur.2022.921705. eCollection 2022. Front Neurol. 2022. PMID: 36212660 Free PMC article. Review. - Evaluation of M2-like macrophage enrichment after diffuse traumatic brain injury through transient interleukin-4 expression from engineered mesenchymal stromal cells.
Enam SF, Kader SR, Bodkin N, Lyon JG, Calhoun M, Azrak C, Tiwari PM, Vanover D, Wang H, Santangelo PJ, Bellamkonda RV. Enam SF, et al. J Neuroinflammation. 2020 Jun 20;17(1):197. doi: 10.1186/s12974-020-01860-y. J Neuroinflammation. 2020. PMID: 32563258 Free PMC article. - Effects of estrogen receptor GPR30 agonist G1 on neuronal apoptosis and microglia polarization in traumatic brain injury rats.
Pan MX, Tang JC, Liu R, Feng YG, Wan Q. Pan MX, et al. Chin J Traumatol. 2018 Aug;21(4):224-228. doi: 10.1016/j.cjtee.2018.04.003. Epub 2018 May 18. Chin J Traumatol. 2018. PMID: 30017543 Free PMC article. - Oxiracetam ameliorates cognitive deficits in vascular dementia rats by regulating the expression of neuronal apoptosis/autophagy-related genes associated with the activation of the Akt/mTOR signaling pathway.
Xu J, Qi Q, Lv P, Dong Y, Jiang X, Liu Z. Xu J, et al. Braz J Med Biol Res. 2019 Nov 7;52(11):e8371. doi: 10.1590/1414-431X20198371. eCollection 2019. Braz J Med Biol Res. 2019. PMID: 31721903 Free PMC article. - HDAC Inhibition by Valproic Acid Induces Neuroprotection and Improvement of PD-like Behaviors in LRRK2 R1441G Transgenic Mice.
Kim T, Song S, Park Y, Kang S, Seo H. Kim T, et al. Exp Neurobiol. 2019 Aug 31;28(4):504-515. doi: 10.5607/en.2019.28.4.504. Exp Neurobiol. 2019. PMID: 31495079 Free PMC article.
References
- Rosenfeld JV, et al. Early management of severe traumatic brain injury. Lancet. 2012;380(9847):1088–1098. - PubMed
- Spitz G, Maller JJ, O’Sullivan R, Ponsford JL. White matter integrity following traumatic brain injury: The association with severity of injury and cognitive functioning. Brain Topogr. 2013;26(4):648–660. - PubMed
- Betz J, Zhuo J, Roy A, Shanmuganathan K, Gullapalli RP. Prognostic value of diffusion tensor imaging parameters in severe traumatic brain injury. J Neurotrauma. 2012;29(7):1292–1305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS43802/NS/NINDS NIH HHS/United States
- NS45048/NS/NINDS NIH HHS/United States
- NS45287/NS/NINDS NIH HHS/United States
- R01 NS045287/NS/NINDS NIH HHS/United States
- R01 NS043802/NS/NINDS NIH HHS/United States
- R01 NS036736/NS/NINDS NIH HHS/United States
- R01 NS045048/NS/NINDS NIH HHS/United States
- NS36736/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials