Lysophosphatidic Acid signaling in the nervous system - PubMed (original) (raw)
Review
Lysophosphatidic Acid signaling in the nervous system
Yun C Yung et al. Neuron. 2015.
Erratum in
- Neuron. 2015 Apr 8;86(1):341
Abstract
The brain is composed of many lipids with varied forms that serve not only as structural components but also as essential signaling molecules. Lysophosphatidic acid (LPA) is an important bioactive lipid species that is part of the lysophospholipid (LP) family. LPA is primarily derived from membrane phospholipids and signals through six cognate G protein-coupled receptors (GPCRs), LPA1-6. These receptors are expressed on most cell types within central and peripheral nervous tissues and have been functionally linked to many neural processes and pathways. This Review covers a current understanding of LPA signaling in the nervous system, with particular focus on the relevance of LPA to both physiological and diseased states.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
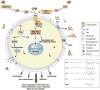
Figure 1. LPA is a bioactive lipid that signals through defined GPCRs within the nervous system
Lipids comprise a significant portion of the central nervous system (CNS) and have differing structural, energetic, and bioactive signaling properties. Signaling lipids are often bound to carrier proteins such as albumin or heat shock proteins. LPA activates members of a family of G protein-coupled receptors (GPCRs) and influences multiple cellular processes including proliferation, survival, apoptosis, morphological change, and migration, as well as the production of other lipids such as prostaglandins through arachidonic acid (AA) conversion by cyclooxygenase-2 (COX-2). The synthetic pathways for LPA include conversion of phosphatidylcholine (PC) into lysophosphatidylcholine (LPC) by lecithin-cholesterol acyltransferase (LCAT) and phospholipase A (PLA) 1 enzymes, or by conversion of PC to phosphatidic acid (PA) by phospholipase D (PLD). LPC is then metabolized to produce lysophosphatidic acid (LPA) by the enzyme autotaxin (ATX). LPA can be broken down into monoacylglycerol (MAG) by a family of lipid phosphate phosphatases (LPPs). Chemical structures are shown to highlight acyl chain composition but do not reflect actual 3-D geometries. Other lipids in the CNS include the endocannabinoid family, fatty acids, cholesterol, and prostaglandins, which are beyond the scope of this review.
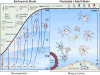
Figure 2. LPAR subtypes in the developing and mature cerebral cortex
Reported LPAR subtype expression of the six LPA receptors, LPA1-6, varies with developmental age and cell type. (Left) LPARs are expressed in neural progenitor cells (NPCs) and other developing cortical cells. These expression patterns vary as the progenitors arise in the ventricular zone (VZ) and differentiate as they migrate through the subventricular zone (SVZ) and intermediate zone (IZ), to localize within the cortical plate (CP). In the embryonic brain, LPA-mediated processes include proliferation, interkinetic nuclear migration, neurite retraction, survival, morphological change, and cell migration. (Right) Most major cell types in the mature cortex express specific subtypes of LPARs. LPARs are also expressed in cells of the ependyma, blood-brain barrier, and meninges, which overlie the most superficial marginal zone (MZ). Postnatally, LPA signaling influences myelination, microglial and astrocytic responses, vascular stabilization, and higher cognitive processes.
Figure 3. Dysregulated LPA signaling may lead to nervous system disorders
Aberrant LPA signaling, whether produced by overactivation, altered LPA production/degradation, or changes in receptor expression, can disrupt the nervous system to produce sequelae relevant to human brain disorders. (A) LPA signaling has been linked to post-hemorrhagic hydrocephalus. A mouse model recapitulates multiple histological comorbidities seen in humans, including ventriculomegaly, thinning of the cortical plate (CP), formation of neurorosettes, disrupted NPCs within the ventricular zone (VZ) and intermediate zone (IZ), loss of cell adhesion that leads to the presence of free-floating cells in the CSF, compromised ependymal lining, and ventricular occlusions. Hydrocephalus is often chronic, with increased CSF pressure, ventriculomegaly, and decreased brain mass persisting throughout postnatal life. (B) _Lpar1_-null mice display dysregulated neural signaling, with disruption of glutamatergic (AMPA and NMDA receptor expression and composition) and GABAergic (GABA+/PV+ neuron decreases) function, leading to significant cognitive impairments in animal models. (C) Nerve damage induces the production of LPA via ATX-mediated conversion of LPC. LPA stimulates LPA3 on activated microglia, resulting in feed-forward LPA release that, in turn, can activate LPA1 on Schwann cells, leading to downregulation of myelin proteins, progressive demyelination, and initiation of neuropathic pain. (D) Brain tumors often overexpress ATX and LPARs, leading to altered LPAR signaling. LPA1-3 stimulation by increased environmental LPA induces cell migration and promotes cancer cell metastasis.
Similar articles
- Lysophosphatidic acid signalling in development.
Sheng X, Yung YC, Chen A, Chun J. Sheng X, et al. Development. 2015 Apr 15;142(8):1390-5. doi: 10.1242/dev.121723. Development. 2015. PMID: 25852197 Free PMC article. Review. - Biological effects of lysophosphatidic acid in the nervous system.
Frisca F, Sabbadini RA, Goldshmit Y, Pébay A. Frisca F, et al. Int Rev Cell Mol Biol. 2012;296:273-322. doi: 10.1016/B978-0-12-394307-1.00005-9. Int Rev Cell Mol Biol. 2012. PMID: 22559941 Review. - Lysophosphatidic acid in neural signaling.
Ye X, Fukushima N, Kingsbury MA, Chun J. Ye X, et al. Neuroreport. 2002 Dec 3;13(17):2169-75. doi: 10.1097/00001756-200212030-00002. Neuroreport. 2002. PMID: 12488791 Review. - Neurobiology of lysophosphatidic acid signaling.
Fukushima N, Ye X, Chun J. Fukushima N, et al. Neuroscientist. 2002 Dec;8(6):540-50. doi: 10.1177/1073858402238513. Neuroscientist. 2002. PMID: 12467376 Review. - Plasticity-related genes (PRGs/LRPs): a brain-specific class of lysophospholipid-modifying proteins.
Bräuer AU, Nitsch R. Bräuer AU, et al. Biochim Biophys Acta. 2008 Sep;1781(9):595-600. doi: 10.1016/j.bbalip.2008.04.004. Epub 2008 Apr 22. Biochim Biophys Acta. 2008. PMID: 18472022 Review.
Cited by
- Spatial regulation of NMN supplementation on brain lipid metabolism upon subacute and sub-chronic PM exposure in C57BL/6 mice.
Jiang Y, Li F, Ye L, Zhang R, Chen S, Peng H, Zhang H, Li D, Chen L, Zeng X, Dong G, Xu W, Liao C, Zhang R, Luo Q, Chen W. Jiang Y, et al. Part Fibre Toxicol. 2024 Sep 9;21(1):35. doi: 10.1186/s12989-024-00597-3. Part Fibre Toxicol. 2024. PMID: 39252011 Free PMC article. - An aging-sensitive compensatory secretory phospholipase that confers neuroprotection and cognitive resilience.
Vicidomini C, Goode TD, McAvoy KM, Yu R, Beveridge CH, Iyer SN, Victor MB, Leary N, Evans L, Steinbaugh MJ, Lai ZW, Lyon MC, Silvestre MRFS, Bonilla G, Sadreyev RI, Walther TC, Sui SH, Saido T, Yamamoto K, Murakami M, Tsai LH, Chopra G, Sahay A. Vicidomini C, et al. bioRxiv [Preprint]. 2024 Sep 3:2024.07.26.605338. doi: 10.1101/2024.07.26.605338. bioRxiv. 2024. PMID: 39211220 Free PMC article. Preprint. - Gintonin, a Ginseng-Derived Exogenous Lysophosphatidic Acid Receptor Ligand, Protects Astrocytes from Hypoxic and Re-oxygenation Stresses Through Stimulation of Astrocytic Glycogenolysis.
Choi SH, Kim HJ, Cho HJ, Park SD, Lee NE, Hwang SH, Cho IH, Hwang H, Rhim H, Kim HC, Nah SY. Choi SH, et al. Mol Neurobiol. 2019 May;56(5):3280-3294. doi: 10.1007/s12035-018-1308-1. Epub 2018 Aug 16. Mol Neurobiol. 2019. PMID: 30117105 - Identifying mRNAs Residing in Myelinating Oligodendrocyte Processes as a Basis for Understanding Internode Autonomy.
Gould R, Brady S. Gould R, et al. Life (Basel). 2023 Apr 4;13(4):945. doi: 10.3390/life13040945. Life (Basel). 2023. PMID: 37109474 Free PMC article. - LPA1 Receptor Promotes Progesterone Receptor Phosphorylation through PKCα in Human Glioblastoma Cells.
Valdés-Rives SA, Arcos-Montoya D, de la Fuente-Granada M, Zamora-Sánchez CJ, Arias-Romero LE, Villamar-Cruz O, Camacho-Arroyo I, Pérez-Tapia SM, González-Arenas A. Valdés-Rives SA, et al. Cells. 2021 Apr 4;10(4):807. doi: 10.3390/cells10040807. Cells. 2021. PMID: 33916643 Free PMC article.
References
- Annabi B, Lachambre MP, Plouffe K, Sartelet H, Beliveau R. Modulation of invasive properties of CD133+ glioblastoma stem cells: a role for MT1-MMP in bioactive lysophospholipid signaling. Molecular carcinogenesis. 2009;48:910–919. - PubMed
- Aoki J. Mechanisms of lysophosphatidic acid production. Seminars in Cell and Developmental Biology. 2004;15:477–489. - PubMed
- Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. The Journal of biological chemistry. 2002;277:48737–48744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 NS082092/NS/NINDS NIH HHS/United States
- R01 NS084398/NS/NINDS NIH HHS/United States
- R01 MH051699/MH/NIMH NIH HHS/United States
- T32 GM007752/GM/NIGMS NIH HHS/United States
- MH051699/MH/NIMH NIH HHS/United States
- NS082092/NS/NINDS NIH HHS/United States
- NS084398/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous