Molecular basis of Klotho: from gene to function in aging - PubMed (original) (raw)
Review
Molecular basis of Klotho: from gene to function in aging
Yuechi Xu et al. Endocr Rev. 2015 Apr.
Abstract
The discovery of the Klotho (KL) gene, which was originally identified as a putative aging-suppressor gene, has generated tremendous interest and has advanced understanding of the aging process. In mice, the overexpression of the KL gene extends the life span, whereas mutations to the KL gene shorten the life span. The human KL gene encodes the α-Klotho protein, which is a multifunctional protein that regulates the metabolism of phosphate, calcium, and vitamin D. α-Klotho also may function as a hormone, although the α-Klotho receptor(s) has not been found. Point mutations of the KL gene in humans are associated with hypertension and kidney disease, which suggests that α-Klotho may be essential to the maintenance of normal renal function. Three α-Klotho protein types with potentially different functions have been identified: a full-length transmembrane α-Klotho, a truncated soluble α-Klotho, and a secreted α-Klotho. Recent evidence suggests that α-Klotho suppresses the insulin and Wnt signaling pathways, inhibits oxidative stress, and regulates phosphatase and calcium absorption. In this review, we provide an update on recent advances in the understanding of the molecular, genetic, biochemical, and physiological properties of the KL gene. Specifically, this review focuses on the structure of the KL gene and the factors that regulate KL gene transcription, the key sites in the regulation of α-Klotho enzyme activity, the α-Klotho signaling pathways, and the molecular mechanisms that underlie α-Klotho function. This current understanding of the molecular biology of the α-Klotho protein may offer new insights into its function and role in aging.
Figures
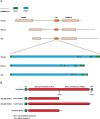
Figure 1.. Architecture of the Klotho gene and protein from the human, mouse, and rat.
A, The structure of the Klotho gene from the human, mouse, and rat. The upstream and downstream neighboring genes are highlighted in light brown. Introns and exons are highlighted in blue and green, respectively. B, Architecture of the human Klotho protein. Protein models were examined using the NCBI Conserved Domains and Protein Classification server.
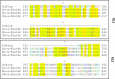
Figure 2.. Structure of the Klotho promoter.
A comparison of the structure of the human (A) and mouse (B) Klotho promoter regions. The match program was used to analyze the transcription factor binding sites (underlined). The EMBOSS CpGPlot/CpGReport/Isochore program was used to screen the CpG islands (highlighted in blue). The transcription coding sequences are indicated in uppercase italic characters.
Figure 3.. Sequence alignment of selective Klotho sequences.
aa are colored according to their chemistry (blue = acidic; red = G or P; dark red = basic; green = hydrophobic), and conserved positions are highlighted when their aa occupants conform predominantly to a chemical type. The active site “E” is labeled with an asterisk (*). Positions of the starting aa in the total proteins are listed.
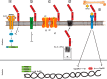
Figure 4.. Overview of the functions of Klotho.
a, Klotho inhibits the IGF signaling pathway. The downstream factors FOXO1, -3a, and -4 mediate the function of Klotho. b, Klotho suppresses the Wnt signaling pathway. c, Klotho regulates Trpv5 calcium channels. d, Klotho regulates PTH synthesis. e, The Klotho-FGF23 complex regulates Pi absorption, mineral metabolism, and vitamin D3 expression and activity.
Similar articles
- Klotho gene, phosphocalcic metabolism, and survival in dialysis.
Torres PU, Prié D, Beck L, De Brauwere D, Leroy C, Friedlander G. Torres PU, et al. J Ren Nutr. 2009 Jan;19(1):50-6. doi: 10.1053/j.jrn.2008.10.018. J Ren Nutr. 2009. PMID: 19121771 Review. - The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice.
Chen TH, Kuro-O M, Chen CH, Sue YM, Chen YC, Wu HH, Cheng CY. Chen TH, et al. Eur J Pharmacol. 2013 Jan 5;698(1-3):67-73. doi: 10.1016/j.ejphar.2012.09.032. Epub 2012 Oct 3. Eur J Pharmacol. 2013. PMID: 23041151 - Klotho as a regulator of oxidative stress and senescence.
Kuro-o M. Kuro-o M. Biol Chem. 2008 Mar;389(3):233-41. doi: 10.1515/BC.2008.028. Biol Chem. 2008. PMID: 18177265 Review. - The FGF23/KLOTHO Regulatory Network and Its Roles in Human Disorders.
Kinoshita S, Kawai M. Kinoshita S, et al. Vitam Horm. 2016;101:151-74. doi: 10.1016/bs.vh.2016.02.001. Epub 2016 Mar 2. Vitam Horm. 2016. PMID: 27125741 Review. - Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism.
Kuro-o M. Kuro-o M. Curr Opin Nephrol Hypertens. 2006 Jul;15(4):437-41. doi: 10.1097/01.mnh.0000232885.81142.83. Curr Opin Nephrol Hypertens. 2006. PMID: 16775459 Review.
Cited by
- Uric acid mediates the association between testosterone and α-Klotho among males: results from the NHANES 2013-2016.
Guo A, Cao J, Wu C, Ding S. Guo A, et al. Int Urol Nephrol. 2025 Mar;57(3):939-946. doi: 10.1007/s11255-024-04262-8. Epub 2024 Nov 2. Int Urol Nephrol. 2025. PMID: 39487906 - Involvement of Single Nucleotide Variants in the Klotho Gene Among Obesity Individuals with and without Type 2 Diabetes Mellitus in the Saudi Population.
Alageel AA, Ali Khan I. Alageel AA, et al. Diabetes Metab Syndr Obes. 2024 Sep 29;17:3603-3617. doi: 10.2147/DMSO.S473843. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39363894 Free PMC article. - The effect of Klotho protein complexed with nanomaterials on bone mesenchymal stem cell performance in the treatment of diabetic ischaemic ulcer.
Tang R, Zhao G, Wang Y, Zhang R. Tang R, et al. IET Nanobiotechnol. 2022 Dec;16(9):316-324. doi: 10.1049/nbt2.12099. Epub 2022 Sep 26. IET Nanobiotechnol. 2022. PMID: 36161768 Free PMC article. - Role of Rho in Salt-Sensitive Hypertension.
Kawarazaki W, Fujita T. Kawarazaki W, et al. Int J Mol Sci. 2021 Mar 15;22(6):2958. doi: 10.3390/ijms22062958. Int J Mol Sci. 2021. PMID: 33803946 Free PMC article. Review. - The potential therapeutic effect of klotho on cell viability in human colorectal adenocarcinoma HT-29 cells.
Sariboyaci AE, Uysal O, Soykan MN, Gunes S. Sariboyaci AE, et al. Med Oncol. 2022 Sep 7;39(12):191. doi: 10.1007/s12032-022-01793-x. Med Oncol. 2022. PMID: 36071274
References
- Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. - PubMed
- Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev. 2005;126:1274–1283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG049780/AG/NIA NIH HHS/United States
- R01 HL102074/HL/NHLBI NIH HHS/United States
- R01 HL118558/HL/NHLBI NIH HHS/United States
- R01 HL105302/HL/NHLBI NIH HHS/United States
- R01 DK093403/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical