A balance between membrane elasticity and polymerization energy sets the shape of spherical clathrin coats - PubMed (original) (raw)
A balance between membrane elasticity and polymerization energy sets the shape of spherical clathrin coats
Mohammed Saleem et al. Nat Commun. 2015.
Abstract
In endocytosis, scaffolding is one of the mechanisms to create membrane curvature by moulding the membrane into the spherical shape of the clathrin cage. However, the impact of membrane elastic parameters on the assembly and shape of clathrin lattices has never been experimentally evaluated. Here, we show that membrane tension opposes clathrin polymerization. We reconstitute clathrin budding in vitro with giant unilamellar vesicles (GUVs), purified adaptors and clathrin. By changing the osmotic conditions, we find that clathrin coats cause extensive budding of GUVs under low membrane tension while polymerizing into shallow pits under moderate tension. High tension fully inhibits polymerization. Theoretically, we predict the tension values for which transitions between different clathrin coat shapes occur. We measure the changes in membrane tension during clathrin polymerization, and use our theoretical framework to estimate the polymerization energy from these data. Our results show that membrane tension controls clathrin-mediated budding by varying the membrane budding energy.
Figures
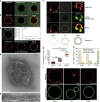
Figure 1. Reconstitution of clathrin polymerization on GUVs and effects of osmotic shocks.
(a) GUVs labelled with 1 mol% TMR-PIP2 (red) incubated with AF488-clathrin alone (green) for 5 min. (b) Same conditions as in a but with AP180/AF488-clathrin. (c) Fluorescence recovery after photobleaching experiments of the AF488-clathrin coat on the membrane (see text). Scale bars, 5 μm (a–c). (d,e) TEM micrographs of GUVs coated with clathrin (scale bars, 200 nm (d) and 100 nm (e)). (f) GUVs labelled with TMR-PIP2 (red) were incubated with AP180/AF488-clathrin in three osmotic conditions: hypotonic (hypo), isotonic (iso) and hypertonic (hyper). Scale bar, 5 μm. (g) Mean fluorescence intensity of the clathrin binding (see Methods) for each osmotic condition, with s.e. (***P<0.0001 in _t_-test). (h) Statistics of vesicle appearance (large, small or no deformation) for the three osmotic conditions. (i) GUVs labelled with TMR-PIP2 (red) were incubated with AF488-AP180 under various osmotic conditions—hypotonic (hypo), isotonic (iso) and hypertonic (hyper). Scale bar, 5 μm. For all the fluorescence experiments, _n_=30–45 under each condition from at least three independent experiments.
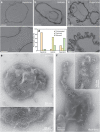
Figure 2. TEM images of GUVs incubated with AP180/clathrin under different osmotic conditions.
(a) Hypotonic conditions. Scale bars, 1 μm (top row) and 200 nm (bottom row). (b) Isotonic conditions. Scale bar, 200 nm. (c) Hypertonic conditions. Scale bars, 1 μm (top row) and 200 nm (bottom row). (d) Statistics of GUVs appearance observed by TEM under different conditions. (e) Micrographs of clathrin lattices in isotonic conditions (scale bar, 200 nm) with a high-magnification image (scale bar, 100 nm (bottom)) and (f) micrographs of clathrin lattices in hypertonic conditions showing tabulation of the membrane (scale bar, 200 nm) with a high-magnification image (inset, scale bar, 100 nm). For electron microscopy experiments, _n_=372 (hypotonic), _n_=54 (isotonic) and _n_=134 (hypertonic) from at least three independent experiments.
Figure 3. Model and clathrin polymerization energy measurements.
(a) The four possible states of a single bud considered in the model: bare membrane (no clathrin binding), shallow partial budding, deep partial budding and full budding (yielding a closed bud). Parameterization of the bud is shown on the deep partial bud as discussed in the text. (b) Our mathematical model predicts the state of a membrane densely packed with contiguous buds. Our fitting of the model parameters (see main text) predicts a bud depth ≃2 nm. (c) Phase diagram predicted by our model, showing the predicted budding state as a function of the scaled tension and polymerization energy (logarithmic plot). (d) Experimental set-up for the measurement of the clathrin polymerization energy: OT, optical tweezers; GUV, giant unilamellar vesicle. (e) Confocal images of AF488-clathrin and TMR-PIP2 channel (right images) before and after clathrin injection (inverted contrast). Scale bar, 10 μm. (f) Plot showing tube force versus time during addition of clathrin. (g) Three-dimensional phase diagram with experimental data from e and f (linear plot). The clathrin polymerization energy was measured from 15 independent experiments.
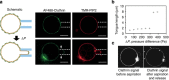
Figure 4. Clathrin coat rupture experiments.
(a) Schematic and confocal images of AP180/AF488-clathrin-coated GUVs before and after being aspirated using a pipette. Arrows show the clathrin-uncoated part of the membrane tongue. (b) Length of the tongue versus aspiration pressure (Δ_P_). (c) AF488-clathrin before and after aspiration (tongue fully released). Arrowheads indicate cracks in the clathrin coat. Scale bars, 5 μm. The experiment was repeated 12 times to measure the average aspiration pressure Δ_P_ for coat rupture.
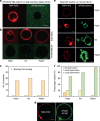
Figure 5. Effect of high bending rigidity and epsin on the clathrin budding abilities.
(a) AF488-clathrin/AP180 does not bind to GUVs with high bending rigidity (see Methods) under isotonic conditions. (b) AF488-clathrin does not bind to high bending rigidity GUVs in hypo-, iso- and hypertonic conditions. (c) Number of vesicles showing AP180/clathrin binding or no binding under various external buffer conditions. (d) A high binding rigidity did not preclude binding of AP180. (e) AF488-clathrin binding and membrane deformation in the presence of unlabelled epsin were observed under varying osmotic conditions, except in the hypotonic conditions where a fraction of the vesicles remained uncoated. (f) Percentage of vesicles with no, small or large deformation after AF488-clathrin/epsin binding. Scale bar, 5 μm. For all the fluorescence experiments, _n_=30–45 under each condition from at least three independent experiments.
Similar articles
- Rule-based modelling provides an extendable framework for comparing candidate mechanisms underpinning clathrin polymerisation.
Sorokin A, Heil KF, Armstrong JD, Sorokina O. Sorokin A, et al. Sci Rep. 2018 Apr 4;8(1):5658. doi: 10.1038/s41598-018-23829-x. Sci Rep. 2018. PMID: 29618727 Free PMC article. - Curvature of clathrin-coated pits driven by epsin.
Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Ford MG, et al. Nature. 2002 Sep 26;419(6905):361-6. doi: 10.1038/nature01020. Nature. 2002. PMID: 12353027 - ENDOCYTOSIS. Endocytic sites mature by continuous bending and remodeling of the clathrin coat.
Avinoam O, Schorb M, Beese CJ, Briggs JA, Kaksonen M. Avinoam O, et al. Science. 2015 Jun 19;348(6241):1369-72. doi: 10.1126/science.aaa9555. Science. 2015. PMID: 26089517 - Clathrin: anatomy of a coat protein.
Smith CJ, Pearse BM. Smith CJ, et al. Trends Cell Biol. 1999 Sep;9(9):335-8. doi: 10.1016/s0962-8924(99)01631-1. Trends Cell Biol. 1999. PMID: 10461185 Review. - Clathrin-coated vesicle formation and protein sorting: an integrated process.
Schmid SL. Schmid SL. Annu Rev Biochem. 1997;66:511-48. doi: 10.1146/annurev.biochem.66.1.511. Annu Rev Biochem. 1997. PMID: 9242916 Review.
Cited by
- Branched actin networks are organized for asymmetric force production during clathrin-mediated endocytosis in mammalian cells.
Jin M, Shirazinejad C, Wang B, Yan A, Schöneberg J, Upadhyayula S, Xu K, Drubin DG. Jin M, et al. Nat Commun. 2022 Jun 22;13(1):3578. doi: 10.1038/s41467-022-31207-5. Nat Commun. 2022. PMID: 35732852 Free PMC article. - Sterols lower energetic barriers of membrane bending and fission necessary for efficient clathrin-mediated endocytosis.
Anderson RH, Sochacki KA, Vuppula H, Scott BL, Bailey EM, Schultz MM, Kerkvliet JG, Taraska JW, Hoppe AD, Francis KR. Anderson RH, et al. Cell Rep. 2021 Nov 16;37(7):110008. doi: 10.1016/j.celrep.2021.110008. Cell Rep. 2021. PMID: 34788623 Free PMC article. - Relaxation of Loaded ESCRT-III Spiral Springs Drives Membrane Deformation.
Chiaruttini N, Redondo-Morata L, Colom A, Humbert F, Lenz M, Scheuring S, Roux A. Chiaruttini N, et al. Cell. 2015 Nov 5;163(4):866-79. doi: 10.1016/j.cell.2015.10.017. Epub 2015 Oct 29. Cell. 2015. PMID: 26522593 Free PMC article. - Physicochemical considerations for bottom-up synthetic biology.
Śmigiel WM, Lefrançois P, Poolman B. Śmigiel WM, et al. Emerg Top Life Sci. 2019 Nov 11;3(5):445-458. doi: 10.1042/ETLS20190017. Emerg Top Life Sci. 2019. PMID: 33523159 Free PMC article. - Endocytosis at extremes: Formation and internalization of giant clathrin-coated pits under elevated membrane tension.
Akatay AA, Wu T, Djakbarova U, Thompson C, Cocucci E, Zandi R, Rudnick J, Kural C. Akatay AA, et al. Front Mol Biosci. 2022 Sep 21;9:959737. doi: 10.3389/fmolb.2022.959737. eCollection 2022. Front Mol Biosci. 2022. PMID: 36213118 Free PMC article.
References
- Kirchhausen T. Bending membranes. Nat. Cell Biol. 14, 906–908 (2012). - PubMed
- Ford M. G. et al.. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051–1055 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources