Production of Gene-Corrected Adult Beta Globin Protein in Human Erythrocytes Differentiated from Patient iPSCs After Genome Editing of the Sickle Point Mutation - PubMed (original) (raw)
Production of Gene-Corrected Adult Beta Globin Protein in Human Erythrocytes Differentiated from Patient iPSCs After Genome Editing of the Sickle Point Mutation
Xiaosong Huang et al. Stem Cells. 2015 May.
Abstract
Human induced pluripotent stem cells (iPSCs) and genome editing provide a precise way to generate gene-corrected cells for disease modeling and cell therapies. Human iPSCs generated from sickle cell disease (SCD) patients have a homozygous missense point mutation in the HBB gene encoding adult β-globin proteins, and are used as a model system to improve strategies of human gene therapy. We demonstrate that the CRISPR/Cas9 system designer nuclease is much more efficient in stimulating gene targeting of the endogenous HBB locus near the SCD point mutation in human iPSCs than zinc finger nucleases and TALENs. Using a specific guide RNA and Cas9, we readily corrected one allele of the SCD HBB gene in human iPSCs by homologous recombination with a donor DNA template containing the wild-type HBB DNA and a selection cassette that was subsequently removed to avoid possible interference of HBB transcription and translation. We chose targeted iPSC clones that have one corrected and one disrupted SCD allele for erythroid differentiation assays, using an improved xeno-free and feeder-free culture condition we recently established. Erythrocytes from either the corrected or its parental (uncorrected) iPSC line were generated with similar efficiencies. Currently ∼6%-10% of these differentiated erythrocytes indeed lacked nuclei, characteristic of further matured erythrocytes called reticulocytes. We also detected the 16-kDa β-globin protein expressed from the corrected HBB allele in the erythrocytes differentiated from genome-edited iPSCs. Our results represent a significant step toward the clinical applications of genome editing using patient-derived iPSCs to generate disease-free cells for cell and gene therapies. Stem Cells 2015;33:1470-1479.
Keywords: Erythroid cells; Genome editing; Globin switching; Human iPSCs.
© 2015 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures
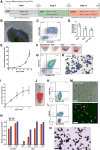
Figure 1. A feeder- and xeno-free culture condition for the hematopoietic differentiation and terminal maturation of human iPSCs
(A) A three-step differentiation strategy from iPSCs to hemoglobinized and enucleated erythrocytes, including spin-EB mediated hematopoietic differentiation (EB), erythroid differentiation (ED), and erythroid terminal maturation (TM). (B–D) Characterization of EB day 14 cells of TNC1 iPSC cultures. (B) Phase-contrast image (10×) of an EB and surrounding hematopoietic cells. Scale bar: 200 μm. (C) Representative flow cytometric analysis of suspension cells harvested EB14 cells that co-express CD34 and CD45. The gates in all flow plots were set based on corresponding fluorescent isotype controls. (D) The yield of CD34+, CD45+, and CD34+CD45+ cells derived from one input iPSC. Means ± s.d., n = 4. (E–H) Erythroid differentiation (ED) step resulting in erythroid commitment, and erythroblast formation and expansion. (E) The accumulated expansion of cell numbers during the ED step. Means ± s.d., n = 3. (F) Gradual color changes of cell pellets along the ED step. (G) A representative flow cytometric plot indicates that the majority of the ED10 cells express immature erythrocyte (erythroblast) markers CD235a and CD36. (H) Wright-Giemsa staining of ED10 cells. Scale bar: 50 μm. (I-O) Erythroid terminal maturation (TM) of TNC1 iPSC derivatives. (I) Cell expansion during the TM step. Means ± s.d., n = 3. (J) Suspension of 90 million TM day 8 (TM8) cells in 1 ml PBS. (K, L) Representative flow cytometric plots of TM8 cells, with >98% cells co-express CD235a and Band3, and ~10% enucleation marked by negative staining of DRAQ5. (M) Phase-contrast and fluorescent images of TM8 cells. Blue arrows indicate enucleated erythrocytes. Scale bars: 30 μm. (N) Quantitative PCR analysis of gene expression of HBB (adult) and HBG (fetal) at different differentiation stages of TNC1 cells, comparing to the cultures of CB CD34+ cells. Means ± s.d., n = 3. (O) benzidine (brown color) for hemoglobin and Wright-Giemsa (nuclear DNA) staining of TM8 cells. 40× image. Scale bar: 50 μm. Blue arrows indicate enucleated erythrocytes lacking nucleus.
Figure 2. A strategy for site-specific correction of the sickle cell disease mutation in the HBB locus using homologous recombination stimulated by Cas9 and a guide RNA
(A) The HBB genomic structure (first two exons), and locations of SCD mutation and gRNA-a targeting HBB exon 1. (B) The gene-targeting donor MGH vector with 2 homology arms (900-bp left arm and 700-bp right arm) introduces a homologous recombination (HR) template for T-to-A replacement in the sickle allele and a loxP-flanked drug-selection cassette PGK-puromycin (puro) to be inserted into the HBB intron 1. (C) Targeted allele following HR with corrected SCD mutation and inserted PGK-puro cassette in intron 1. We used 2 PCR primers (black arrows: TP1F/TP1R and TP2F/TP2R) for initial screening of targeted insertion (TI) indicative of correct HR. (D) Cre-mediated excision removes the PGK-puro cassette and leaves one copy of the loxP DNA (34-bp) and 84-bp linker DNA in HBB intron 1 of the corrected allele. Genomic DNA PCR with primers gHBB-F/gHBB-R produces a 482-bp product from the targeted allele of “Cre” clones. (E) Genomic DNA PCR with primers gHBB-F/gHBB-R produces a 364-bp product from the unintended sickle allele of original and “Cre” clones. (F) PCR screening for targeted integration (TI) by the primer set TP2F/TP2R that detect 3′ TI (1.4-kb). (G) Genomic PCR using the primer set gHBB-F/gHBB-R that amplify the unintended (sickle) allele but not the targeted allele (it is too long to be amplified by PCR with a short extension time) before Cre-mediated excision. (H) PCR screening of Cre-mediated excision of SC15 clones using primer set gHBB-F/gHBB-R. The 482-bp PCR product emerged after Cre-excision as shown in (D).
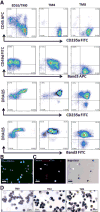
Figure 3. Erythroid terminal maturation of erythroblasts that generated from SC15-Cre2 iPSC clone
The phenotypes of erythroblasts (ED10) from SC15-Cre2 are similar those from TNC1 and BC1 iPSC lines. (A) Cell surface marker expression dynamics during 8 days of terminal maturation (TM). By day 8 of TM (TM8), nearly all the cells expressed CD235a and Band3, and lost the expression of CD45 and CD49d. About 9% of CD235a+ and Band3+ cells were stained negative for DNA by DRAQ5. (B) Fluorescent image of TM8 cells showing Band3 cell surface expression and ~10% lacking DNA staining (red). (C) Fluorescent images of TM8 cells showing cell surface expression CD235a in enucleated cells (lacking blue DAN staining) that are metabolically active based on Calcein AM staining (green, by arrows). (D) Histology staining and morphology of cells after terminal maturation at day 0 (ED10), day 4 (TM4) and day 8 (TM8) after Wright-Giemsa (blue) and benzidine (brown) staining. Enucleated and hemoglobinized cells are easily identified (arrow). Scale bar: 20μm.
Figure 4. Analysis of globin gene and protein expressions the corrected erythrocytes, and their function
(A) Quantitative RT-PCR analysis of HBB and HBG gene expression in iPSCs, after hematopoietic differentiation (EB14), erythroid differentiation (ED10) and terminal maturation (TM8) from SC15-Cre2 and parental TNC1 iPSCs and a control of cord blood (CB) CD34+ cells. (B) Western blot analysis of adult hemoglobin beta (HBB) protein level during the differentiation of iPSC clones before and after correction and excision. The relative levels of full-length HBB proteins normalized to the GAPDH control are also indicated. (C) FACS analysis of intracellular adult and fetal hemoglobin protein expression of maturated erythrocytes generated from SC15-Cre2 iPSC clone. (D) Oxygen affinity curves of erythrocytes from SC15-Cre2 after terminal maturation and a cord blood control as measured by a Hemox Analyzer.
Similar articles
- Both TALENs and CRISPR/Cas9 directly target the HBB IVS2-654 (C > T) mutation in β-thalassemia-derived iPSCs.
Xu P, Tong Y, Liu XZ, Wang TT, Cheng L, Wang BY, Lv X, Huang Y, Liu DP. Xu P, et al. Sci Rep. 2015 Jul 9;5:12065. doi: 10.1038/srep12065. Sci Rep. 2015. PMID: 26156589 Free PMC article. - A Universal Approach to Correct Various HBB Gene Mutations in Human Stem Cells for Gene Therapy of Beta-Thalassemia and Sickle Cell Disease.
Cai L, Bai H, Mahairaki V, Gao Y, He C, Wen Y, Jin YC, Wang Y, Pan RL, Qasba A, Ye Z, Cheng L. Cai L, et al. Stem Cells Transl Med. 2018 Jan;7(1):87-97. doi: 10.1002/sctm.17-0066. Epub 2017 Nov 21. Stem Cells Transl Med. 2018. PMID: 29164808 Free PMC article. - Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease.
Zou J, Mali P, Huang X, Dowey SN, Cheng L. Zou J, et al. Blood. 2011 Oct 27;118(17):4599-608. doi: 10.1182/blood-2011-02-335554. Epub 2011 Aug 31. Blood. 2011. PMID: 21881051 Free PMC article. - CRISPR/Cas9 system and its applications in human hematopoietic cells.
Hu X. Hu X. Blood Cells Mol Dis. 2016 Nov;62:6-12. doi: 10.1016/j.bcmd.2016.09.003. Epub 2016 Oct 2. Blood Cells Mol Dis. 2016. PMID: 27736664 Review. - Gene correction in patient-specific iPSCs for therapy development and disease modeling.
Jang YY, Ye Z. Jang YY, et al. Hum Genet. 2016 Sep;135(9):1041-58. doi: 10.1007/s00439-016-1691-5. Epub 2016 Jun 2. Hum Genet. 2016. PMID: 27256364 Free PMC article. Review.
Cited by
- CRISPR/Cas9 in the treatment of sickle cell disease (SCD) and its comparison with traditional treatment approaches: a review.
Tariq H, Khurshid F, Khan MH, Dilshad A, Zain A, Rasool W, Jawaid A, Kunwar D, Khanduja S, Akbar A. Tariq H, et al. Ann Med Surg (Lond). 2024 Aug 14;86(10):5938-5946. doi: 10.1097/MS9.0000000000002478. eCollection 2024 Oct. Ann Med Surg (Lond). 2024. PMID: 39359808 Free PMC article. Review. - Precision in Action: The Role of Clustered Regularly Interspaced Short Palindromic Repeats/Cas in Gene Therapies.
Banda A, Impomeni O, Singh A, Baloch AR, Hu W, Jaijyan DK. Banda A, et al. Vaccines (Basel). 2024 Jun 7;12(6):636. doi: 10.3390/vaccines12060636. Vaccines (Basel). 2024. PMID: 38932365 Free PMC article. Review. - Hematopoietic Stem Cell Transplantation in Sickle Cell Disease: A Multidimentional Review.
Rostami T, Rad S, Rostami MR, Mirhosseini SA, Alemi H, Khavandgar N, Janbabai G, Kiumarsi A, Kasaeian A, Mousavi SA. Rostami T, et al. Cell Transplant. 2024 Jan-Dec;33:9636897241246351. doi: 10.1177/09636897241246351. Cell Transplant. 2024. PMID: 38680015 Free PMC article. Review. - Exosomes for CRISPR-Cas9 Delivery: The Cutting Edge in Genome Editing.
Aslan C, Zolbanin NM, Faraji F, Jafari R. Aslan C, et al. Mol Biotechnol. 2023 Nov 27. doi: 10.1007/s12033-023-00932-7. Online ahead of print. Mol Biotechnol. 2023. PMID: 38012525 Review. - CRISPR-Cas Technology as a Revolutionary Genome Editing tool: Mechanisms and Biomedical Applications.
Ebrahimi S, Khosravi MA, Raz A, Karimipoor M, Parvizi P. Ebrahimi S, et al. Iran Biomed J. 2023 Sep 1;27(5):219-46. doi: 10.61186/ibj.27.5.219. Epub 2023 Jun 18. Iran Biomed J. 2023. PMID: 37873636 Free PMC article. Review.
References
- Porteus MH, Carroll D. Gene targeting using zinc finger nucleases. Nature biotechnology. 2005;23(8):967–973. - PubMed
- Porteus MH. Mammalian gene targeting with designed zinc finger nucleases. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;13(2):438–446. - PubMed
- Hockemeyer D, Jaenisch R. Gene targeting in human pluripotent cells. Cold Spring Harbor symposia on quantitative biology. 2010;75:201–209. - PubMed
- Collin J, Lako M. Concise review: putting a finger on stem cell biology: zinc finger nuclease-driven targeted genetic editing in human pluripotent stem cells. Stem cells. 2011;29(7):1021–1033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007814/GM/NIGMS NIH HHS/United States
- U01 HL107446/HL/NHLBI NIH HHS/United States
- T32HL007525-31/HL/NHLBI NIH HHS/United States
- T32 HL007525/HL/NHLBI NIH HHS/United States
- R01 HL073781/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials