Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling - PubMed (original) (raw)
Review
Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling
Adriana Almeida de Jesus et al. Annu Rev Immunol. 2015.
Abstract
Patients with autoinflammatory diseases present with noninfectious fever flares and systemic and/or disease-specific organ inflammation. Their excessive proinflammatory cytokine and chemokine responses can be life threatening and lead to organ damage over time. Studying such patients has revealed genetic defects that have helped unravel key innate immune pathways, including excessive IL-1 signaling, constitutive NF-κB activation, and, more recently, chronic type I IFN signaling. Discoveries of monogenic defects that lead to activation of proinflammatory cytokines have inspired the use of anticytokine-directed treatment approaches that have been life changing for many patients and have led to the approval of IL-1-blocking agents for a number of autoinflammatory conditions. In this review, we describe the genetically characterized autoinflammatory diseases, we summarize our understanding of the molecular pathways that drive clinical phenotypes and that continue to inspire the search for novel treatment targets, and we provide a conceptual framework for classification.
Keywords: IFN-mediated autoinflammatory diseases; IL-1-mediated autoinflammatory diseases; autoinflammatory diseases; cryopyrin-associated periodic syndromes; hereditary fever syndromes; inflammasomes in human disease; macrophage activation syndrome; proteasome-associated autoinflammatory syndromes.
Figures
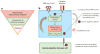
Figure 1
Principles of immune dysregulation in autoinflammatory diseases. (a) Each genetically defined autoinflammatory disease ( yellow) can be classified based on the predominant proinflammatory mediator that is upregulated, where known, (red ) and the component of the innate immune response that is affected by the disease-causing mutations ( green). (b) Dysregulation in four components of the immune response are found to cause autoinflammatory diseases. (Table 1 presents a complete list of genes that are mutated in autoinflammatory diseases and the corresponding components of the innate immune response that are affected.) ❶ Gain-of-function mutations (autosomal dominant, often sporadic/de novo) in genes encoding intracellular PRRs or their adaptor molecules result in constitutively increased innate immune sensor function and increased or continued production of proinflammatory mediators. ❷ Loss-of-function mutations or haploinsufficiency of molecules/enzymes critical for maintaining cell homeostasis can result in accumulation of intracellular stressors that stimulate intracellular sensor/PRR activation and the production of proinflammatory mediators. ❸ Loss-of-function mutations in genes encoding negative regulators that downregulate proinflammatory responses also lead to autoinflammatory diseases. The mutations observed so far lead to loss of function of a cytokine receptor antagonist, or an antiinflammatory cytokine, or failure to terminate the release of inflammatory mediators by inflammatory cells (e.g, cytotoxic dysfunction causing persistent macrophage activation). ❹ Mutations that alter immune receptor signaling cause a group of diseases presenting with often more complex clinical phenotypes that can include autoinflammatory, immunodeficient, and autoimmune features depending on their effect on innate or adaptive immune cells. (Abbreviations: PRR, pattern-recognition receptor; TLR, Toll-like receptor.)
Figure 2
Proposed mechanisms of activation of IL-1-signaling pathways in autoinflammatory diseases. (a) Cryopyrinopathies (CAPS). Unlike wild-type NLRP3, mutated NLRP3 (which causes CAPS) is constitutively activated and thought to oligomerize and bind to the adapter molecule ASC (apoptosis-associated speck-like protein containing a CARD) to form an active catalytic complex with two pro-caspase-1 molecules. Via autocatalysis, this complex generates active caspase-1, which cleaves inactive pro-IL-1β into its active form, IL-1β. (b) Familial Mediterranean fever (FMF). Wild-type pyrin can interact directly with ASC, forming the pyrin inflammasome, which is activated in the presence of FMF-causing mutations. (c) NLRC4-MAS. Mutations in the NACHT domain of NLRC4 cause autoinflammatory diseases that predispose to the development of macrophage-activating syndrome (MAS). The asterisk (*) indicates that caspase-1 activation also leads to IL-18 activation that is highest in NLRC4 inflammasome activation. (d ) Hyper-IgD syndrome (HIDS). Mevalonate kinase (MVK), a critical enzyme in the biosynthesis of sterol and nonsterol isoprenoids, catalyzes the conversion of mevalonate to mevalonate phosphate. In HIDS, activity of this enzyme is reduced, resulting in decreased concentrations of mevalonate phosphate, geranylgeranyl pyrophosphate, and farnesyl pyrophosphate, and impaired geranylgeranylation of a number of proteins. Through an unknown mechanism, the reduced geranylgeranylation would lead to an increased procaspase-1 activation and consequent caspase-1 activation, with resulting overproduction of IL-1β. (e) Majeed syndrome. Lipin-2 catalyzes the conversion of phosphatidate to diacylglycerol, a precursor for the production of phospholipids. Mutations in LPIN2 are thought to lead to an accumulation of fatty acids and intracellular stress that induces inflammasome activation. (f) TNF receptor–associated periodic syndrome (TRAPS). TNFR1 molecules are transported from the endoplasmic reticulum (ER) to the Golgi apparatus and then to the cell surface. Mutated TNFR1 (which causes TRAPS) is misfolded and cannot be transported to the cell surface. Misfolded TNFR1 is sequestered in the ER, where it causes intracellular stress through increased mitochondrial reactive oxygen species (ROS) production that leads to inflammasome activation and increased signaling, including NF-κB activation. ( g) Deficiency of IL-1Ra (DIRA). Deficiency of IL-1Ra leads to unopposed IL-1α and IL-1β signaling. The structure of the inflammasomes was adapted from Reference . Numbers in black circles indicate disease caused by ❶ increased sensor function, ❷ generation of cell stress, or ❸ loss of negative regulator. (Other abbreviations: IL-1Ra, IL-1 receptor antagonist; PYD, pyrin domain.)
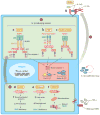
Figure 3
Proposed mechanisms of activation of proinflammatory signaling pathways in IFN-mediated autoinflammatory diseases. Pathways of innate immune sensing of cytosolic nucleotide and stress lead to type I IFN production and a feed-forward loop of IFN signaling. The enzyme cGAS is a cytosolic sensor of dsDNA that, upon activation, generates a small-molecule second messenger, cGAMP, which binds and signals through the adaptor protein STING. Similarly, the RIG-I-like receptor sensors RIG-I and MDA5 are triggered by binding to dsRNA and signal through the adaptor protein MAVS. The common pathway downstream of STING and MAVS includes TBK1 and IRF3 phosphorylation/activation and IFN-β transcription. The disease-causing mutations cause a gain of function of an intracellular sensor (red ) or a loss of function of a protein (blue), leading to generation of intracellular stress. (a) Aicardi-Goutières syndrome 7 (AGS7). MDA5, encoded by IFIH1, is one of the dsRNA sensors. Gain-of-function mutation in MDA5 causes constitutive or enhanced IFN-β transcription, resulting in a clinical syndrome similar to that caused by TREX1 mutation. (b) STING-associated vasculopathy with onset in infancy (SAVI). Gain-of-function mutations in STING cause spontaneous or enhanced transcription of IFN-β, leading to the clinical syndrome SAVI. (c) PRAAS/CANDLE. Genetic mutations in proteasome subunits cause loss of function and cellular stress. Through a still unclear process, reduced proteasome function leads to type I IFN production and the inflammatory disease phenotype of CANDLE. Whether the stress response due to defective proteasome function that is triggered through the cytosolic nucleotide–sensing pathway or other sensors that are coupled to type I IFN production remains unknown. The transcription and secretion of type I IFN results in a cytokine amplification loop in the same cells or other bystander cells. IRF7 is one of the IFN response genes, which further promote type I IFN transcription and amplification of the process. (d ) Aicardi-Goutières syndrome 1–6 (AGS1–6). TREX1 loss-of-function mutations cause accumulation of ssDNA derived from an endogenous retroelement, resulting in STING-dependent type I IFN transcription (AGS1). Similarly, loss-of-function mutations in RNASEH2B, RNASEH2C, RNASEH2A, SAMHD1, and ADAR1 result in type I IFN transcription through still unknown signaling processes (AGS2–6). Numbers in black circles indicate disease caused by ❶ increased sensor/adaptor function or ❷ generation of cell stress. (Other abbreviations: AMP, adenosine monophosphate; CANDLE, chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature; cGAMP, cyclic GMP-AMP; cGAS, cyclic GMP-AMP synthase; GMP, guanosine monophosphate; MAVS, mitochondrial antiviral-signaling protein; PRAAS, proteasome-associated autoinflammatory syndromes; TREX1, three prime repair exonuclease 1.)
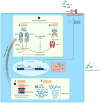
Figure 4
Potential mechanisms of NF-κB-mediated diseases. (a) Pediatric granulomatous arthritis/Blau syndrome. Upon activation or when mutated, NOD2 oligomerizes and recruits the kinase RIPK2. IAP proteins then mediate the ubiquitylation of RIPK2, enabling it to initiate a signal pathway that results in transcriptional activation, most notably through the canonical NF-κB pathway. Oligomerized NOD2 can also associate with the mitochondrial adaptor protein MAVS to activate IFN production. (b) CARD14-mediated psoriasis (CAMPS). Expression of adaptor protein CARD14 is largely restricted to keratinocytes. Although it is unclear what signals drive CARD14 activation, activation of PKC results in CARD14 phosphorylation and activation. Upon activation or when mutated, as in patients with CAMPS, it associates with the BCL10/MALT1 complex, resulting in excessive NF-κB activation. NF-κB-associated gene transcription then drives neutrophil and lymphocyte chemotaxis and psoriasis-like skin inflammation. In general, the inflammatory mediators induced by excessive NF-κB activation vary by disease and cell type. Number in black circled indicates ❶ disease caused by increased sensor/adaptor function. (Other abbreviations: IAP, inhibitor of apoptosis; MAVS, mitochondrial antiviral-signaling protein; PKC, protein kinase C; XIAP, X-linked IAP.)
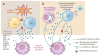
Figure 5
Schematic of the mechanisms resulting in systemic macrophage activation. (a) In familial HLH, cytotoxic lymphocytes lack the ability to kill infected antigen-presenting cells. ➀ This results in failure to clear the infection and failure to terminate their own stimulation. With unrestrained stimulation, these lymphocytes produce extraordinary amounts of macrophage-stimulating cytokines, such as IFN-γ. They also lack the ability to kill activated macrophages. ➁ In MAS, intrinsic defects such as hyperactivity of the NLRC4 inflammasome could prime for macrophage activation directly, ➂ but they could also cause constitutive IL-18 production that is enhanced upon macrophage activation. Upon infection or stress, lymphocytes (e.g., NK cells) chronically exposed to IL-18 could ➃ prime for cytokine overproduction, ➄ impair cytotoxicity, or ➅ promote NK cell death. Systemic macrophage activation results in the release of a variety of potent inflammatory mediators (IL-1β, IL-6, TNF-α, IL-33, IL-1α, HMGB1, S100 proteins, etc.) that cause the shock-like symptoms associated with MAS and HLH. (Abbreviations: HLH, hemophagocytic lymphohistiocytosis; MAS, macrophage activation syndrome; XIAP, X-linked inhibitor of apoptosis.)
Figure 6
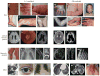
Clinical manifestations of IL-1-mediated and IFN-mediated diseases. (a) Urticarial rash in NOMID. (b,c) Pustular rash in DIRA. (d ) Erysipelas-like erythema in FMF. (e) Erythematous dermal macules (migratory during a disease flare) in a patient with TRAPS. (f) Purpuric rash in a patient with HIDS. ( g) Leptomeningeal enhancement in NOMID. (h) Inflammation-induced chronic papilledema in NOMID. (i ) Hydrocephalus and cerebral atrophy in NOMID. (j ) Patella enlargement in NOMID. (k) Widening of multiple ribs (asterisks) and clavicles (arrows) in DIRA osteomyelitis. (l ) Spine MRI showing destruction of vertebral bodies and severe kyphosis due to osteomyelitis in a patient with DIRA (red arrow indicates collapsed vertebra). (m) Metaphyseal bone overgrowth in NOMID. (n) Cochlear enhancement in NOMID. (o) Conjunctival erythema in a patient with TRAPS. ( p) Pleural effusion in a patient with FMF. (q) Finger and hand swelling in CANDLE syndrome. (r) Erythematous-macular rash in AGS. (s) Characteristic lipodystrophy in a patient with CANDLE syndrome. (t) Erythematous-nodular rash in a patient with CANDLE. (u) Intense plantar erythema/vasculitis in a patient with SAVI. (v) Purpuric and papular rash in a patient with SAVI. (w) Basal ganglia calcifications in CANDLE syndrome. (x) Severe hydrocephalus and cerebral atrophy in AGS. ( y) Patchy myositis in CANDLE syndrome. (z) Evidence of myositis and panniculitis in a bilateral thigh MRI of a CANDLE patient. (aa) Bone resorption in a patient with SAVI. (ab) Interstitial lung disease in a patient with SAVI. (ac) Intra-abdominal fat deposition in a patient with CANDLE syndrome. (ad ) Telangiectasia, atrophy, and scarring of the skin with loss of deep tissue of the nose in a patient with SAVI. (Abbreviations: AGS, Aicardi-Goutières syndrome; CANDLE, chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature; DIRA, deficiency of the IL-1 receptor antagonist; FMF, familial Mediterranean fever; HIDS, hyper-IgD syndrome; IFN, interferon; NOMID, neonatal-onset multisystem inflammatory disease; SAVI, STING-associated vasculopathy with onset in infancy; TRAPS, TNF receptor–associated periodic syndrome.)
Similar articles
- New monogenic autoinflammatory diseases--a clinical overview.
Canna SW, Goldbach-Mansky R. Canna SW, et al. Semin Immunopathol. 2015 Jul;37(4):387-94. doi: 10.1007/s00281-015-0493-5. Epub 2015 May 12. Semin Immunopathol. 2015. PMID: 25963521 Free PMC article. Review. - Monogenic IL-1 mediated autoinflammatory and immunodeficiency syndromes: finding the right balance in response to danger signals.
Henderson C, Goldbach-Mansky R. Henderson C, et al. Clin Immunol. 2010 May;135(2):210-22. doi: 10.1016/j.clim.2010.02.013. Epub 2010 Mar 30. Clin Immunol. 2010. PMID: 20353899 Free PMC article. Review. - Monogenic autoinflammatory disorders: Conceptual overview, phenotype, and clinical approach.
Nigrovic PA, Lee PY, Hoffman HM. Nigrovic PA, et al. J Allergy Clin Immunol. 2020 Nov;146(5):925-937. doi: 10.1016/j.jaci.2020.08.017. J Allergy Clin Immunol. 2020. PMID: 33160483 Free PMC article. Review. - The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation.
Manthiram K, Zhou Q, Aksentijevich I, Kastner DL. Manthiram K, et al. Nat Immunol. 2017 Jul 19;18(8):832-842. doi: 10.1038/ni.3777. Nat Immunol. 2017. PMID: 28722725 Review. - A systematic approach to autoinflammatory syndromes: a spelling booklet for the beginner.
Rigante D. Rigante D. Expert Rev Clin Immunol. 2017 Jun;13(6):571-597. doi: 10.1080/1744666X.2017.1280396. Epub 2017 Feb 1. Expert Rev Clin Immunol. 2017. PMID: 28064547 Review.
Cited by
- Inflammatory turmoil within: an exploration of autoinflammatory disease genetic underpinnings, clinical presentations, and therapeutic approaches.
Kozu KT, Nascimento RRNRD, Aires PP, Cordeiro RA, Moura TCL, Sztajnbok FR, Pereira IA, Almeida de Jesus A, Perazzio SF. Kozu KT, et al. Adv Rheumatol. 2024 Aug 22;64(1):62. doi: 10.1186/s42358-024-00404-9. Adv Rheumatol. 2024. PMID: 39175060 Review. - Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade.
DeSena AD, Do T, Schulert GS. DeSena AD, et al. J Neuroinflammation. 2018 Feb 9;15(1):38. doi: 10.1186/s12974-018-1063-2. J Neuroinflammation. 2018. PMID: 29426321 Free PMC article. - Genetically defined autoinflammatory diseases.
de Jesus AA, Goldbach-Mansky R. de Jesus AA, et al. Oral Dis. 2016 Oct;22(7):591-604. doi: 10.1111/odi.12448. Epub 2016 Apr 14. Oral Dis. 2016. PMID: 26837051 Free PMC article. Review. - Primary immune regulatory disorders: a growing universe of immune dysregulation.
Chan AY, Torgerson TR. Chan AY, et al. Curr Opin Allergy Clin Immunol. 2020 Dec;20(6):582-590. doi: 10.1097/ACI.0000000000000689. Curr Opin Allergy Clin Immunol. 2020. PMID: 32941318 Free PMC article. Review. - Interstitial Lung Disease Caused by STING-associated Vasculopathy with Onset in Infancy.
Clarke SL, Pellowe EJ, de Jesus AA, Goldbach-Mansky R, Hilliard TN, Ramanan AV. Clarke SL, et al. Am J Respir Crit Care Med. 2016 Sep 1;194(5):639-42. doi: 10.1164/rccm.201510-2102LE. Am J Respir Crit Care Med. 2016. PMID: 27585386 Free PMC article. No abstract available.
References
- McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–44. - PubMed
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Part 1):1–13. - PubMed
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. - PubMed
- Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. 2014;32:461–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical