Anti-PEG immunity: emergence, characteristics, and unaddressed questions - PubMed (original) (raw)
Review
. 2015 Sep-Oct;7(5):655-77.
doi: 10.1002/wnan.1339. Epub 2015 Feb 23.
Affiliations
- PMID: 25707913
- PMCID: PMC4515207
- DOI: 10.1002/wnan.1339
Review
Anti-PEG immunity: emergence, characteristics, and unaddressed questions
Qi Yang et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015 Sep-Oct.
Abstract
The modification of protein and nanoparticle therapeutics with polyethylene glycol (PEG), a flexible, uncharged, and highly hydrophilic polymer, is a widely adopted approach to reduce RES clearance, extend circulation time, and improve drug efficacy. Nevertheless, an emerging body of literature, generated by numerous research groups, demonstrates that the immune system can produce antibodies that specifically bind PEG, which can lead to the 'accelerated blood clearance' of PEGylated therapeutics. In animals, anti-PEG immunity is typically robust but short-lived and consists of a predominantly anti-PEG IgM response. Rodent studies suggest that the induction of anti-PEG antibodies (α-PEG Abs) primarily occurs through a type 2 T-cell independent mechanism. Although anti-PEG immunity is less well-studied in humans, the presence of α-PEG Abs has been correlated with reduced efficacy of PEGylated therapeutics in clinical trials. The prevalence of anti-PEG IgG and reports of memory immune responses, as well as the existence of α-PEG Abs in healthy untreated individuals, suggests that the mechanism(s) and features of human anti-PEG immune responses may differ from those of animal models. Many questions, including the incidence rate of pre-existing α-PEG Abs and immunological mechanism(s) of α-PEG Ab formation in humans, must be answered in order to fully address the potential complications of anti-PEG immunity.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Figure 1
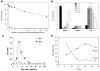
A) The conformation adopted by PEG chains at various grafting densities. At low grafting densities (RF/D ≤ 1), the PEG chains adopt a diffuse “mushroom” conformation. At higher densities, the PEG chains are increasingly able to repel opsonization and cell uptake as they transition into a more extended “brush” conformation (RF/D > 1) and eventually reach a “dense brush” regime (RF/D > 2.8). B) Uptake of PEG5k-grafted gold nanoparticles by mouse J774A.1 macrophage-like cells. A PEG5k density of 0.16 PEG/nm2 corresponds to brush conformation; all other PEG densities correspond to dense brush conformation. C) Qualitative analysis of the serum proteins adsorbed onto 30 nm gold nanoparticles modified with varying amounts of PEG5k. A PEG5k density of 0.24 PEG/nm2 corresponds to brush conformation; all other PEG densities correspond to dense brush conformation. D) Phase diagram mapping polymeric nanoparticle uptake by human THP-1 macrophage-like cells as a function of PEG length (MW) and coating density (PEG groups/nm2). The mushroom, brush, and dense brush conformations are indicated in dark gray, gray, and white, respectively, and the transitions between the mushroom-brush and brush-dense brush conformations are indicated by the dashed and dotted lines, respectively. E) Blood circulation profiles, as observed by intravital microscopy, of 100 nm unmodified (COOH) and PEG5k-grafted polystyrene nanoparticles. Panels B and C were reprinted with permission from Ref (copyright 2012 ACS), and panels A, D, and E were adapted with permission from Ref (copyright 2014 ACS).
Figure 2
A) Amount of 99mTc-labeled PEGylated liposomes remaining in circulation after i.v. administration in rats quantified by scintigraphic image analysis. (●) indicates the first dose, and (○) indicates the second dose given 7 days later. B) Tissue biodistribution of 99mTc-labeled PEGylated liposomes in rats for the initial injection (control) and for second doses given after 7, 14, 21, or 28 days. *p < 0.05, **p < 0.01. C) PEG-specific antibodies responses after an initial injection of PEGylated liposomes (0.001 μmol/kg) in rats, as determined using ELISA. *p < 0.05, ***p < 0.005. D) PEG-specific antibodies responses after an initial injection of PEGylated liposomes (100 μg/animal) in mice, as determined using ELISA. Panels A and B were reprinted from Ref ; panel C was reprinted from Ref with permission from Elsevier; and panel D was reprinted from Ref with permission from Elsevier.
Figure 3
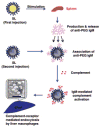
Proposed type-2 T-cell independent (TI-2) response mechanism for the formation of α-PEG Abs and the ABC effect. Splenic B cells are stimulated by an initial dose of PEGylated therapeutic and produce α-PEG IgM. These antibodies then associate with subsequent doses of PEGylated systems and activate complement proteins, which then opsonize PEGylated system and lead to its eventual clearance through hepatic MPS cells. Reprinted from Ref by permission from Macmillan Publishers Ltd.
Figure 4
Anti-PEG IgM vs. asparaginase (ASNase) activity for patients treated with A) PEG-ASNase and B) ASNase. Flow cytometry was used to detect α-PEG Abs bound to PEG hydrogel (TentaGel-OH) particles. C) Mean serum uric acid (sUA) levels in patients receiving biweekly i.v. infusions of PEG-uricase. Normal sUA levels are typically defined as ≤6 mg/dL (indicated by gray dashed line). D) Mean α-PEG Ab titers in patients receiving biweekly i.v. infusions of PEG-uricase. Panels A and B were reprinted from Ref , and panels C and D were modified from Ref .
Figure 5
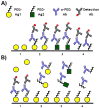
A) Direct and B) competitive enzyme-linked immunosorbent assays (ELISAs) should be used in combination to determine the PEG-specificity of Ab responses induced after treatment with a PEGylated agent (PEG-Ag1), as well as pre-existing α-PEG Abs. In direct ELISAs, PEG specificity can be confirmed by the cross-reactivity of α-PEG Abs to plates coated with pure PEG polymers (see A4) or other PEGylated materials (see A3). In competitive ELISAs, PEG specificity can be confirmed via the inhibition of α-PEG Ab binding by increasing concentrations of free PEG (see B4) or other PEGylated materials (see B3). Additionally, α-PEG Abs should not directly bind to non-PEGylated treatment agents (see A1) nor be competitively inhibited in their presence (see B1).
Similar articles
- Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation.
Zhang P, Sun F, Liu S, Jiang S. Zhang P, et al. J Control Release. 2016 Dec 28;244(Pt B):184-193. doi: 10.1016/j.jconrel.2016.06.040. Epub 2016 Jun 28. J Control Release. 2016. PMID: 27369864 Free PMC article. Review. - Treatment-induced and Pre-existing Anti-peg Antibodies: Prevalence, Clinical Implications, and Future Perspectives.
Gaballa SA, Shimizu T, Ando H, Takata H, Emam SE, Ramadan E, Naguib YW, Mady FM, Khaled KA, Ishida T. Gaballa SA, et al. J Pharm Sci. 2024 Mar;113(3):555-578. doi: 10.1016/j.xphs.2023.11.001. Epub 2023 Nov 4. J Pharm Sci. 2024. PMID: 37931786 Review. - Anti-PEG antibodies alter the mobility and biodistribution of densely PEGylated nanoparticles in mucus.
Henry CE, Wang YY, Yang Q, Hoang T, Chattopadhyay S, Hoen T, Ensign LM, Nunn KL, Schroeder H, McCallen J, Moench T, Cone R, Roffler SR, Lai SK. Henry CE, et al. Acta Biomater. 2016 Oct 1;43:61-70. doi: 10.1016/j.actbio.2016.07.019. Epub 2016 Jul 14. Acta Biomater. 2016. PMID: 27424083 Free PMC article. - Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population.
Yang Q, Jacobs TM, McCallen JD, Moore DT, Huckaby JT, Edelstein JN, Lai SK. Yang Q, et al. Anal Chem. 2016 Dec 6;88(23):11804-11812. doi: 10.1021/acs.analchem.6b03437. Epub 2016 Nov 16. Anal Chem. 2016. PMID: 27804292 Free PMC article. - Anti-PEG IgM Is a Major Contributor to the Accelerated Blood Clearance of Polyethylene Glycol-Conjugated Protein.
Mima Y, Hashimoto Y, Shimizu T, Kiwada H, Ishida T. Mima Y, et al. Mol Pharm. 2015 Jul 6;12(7):2429-35. doi: 10.1021/acs.molpharmaceut.5b00144. Epub 2015 Jun 24. Mol Pharm. 2015. PMID: 26070445
Cited by
- PEG-8 Laurate Fermentation of Staphylococcus epidermidis Reduces the Required Dose of Clindamycin Against Cutibacterium acnes.
Marito S, Keshari S, Huang CM. Marito S, et al. Int J Mol Sci. 2020 Jul 19;21(14):5103. doi: 10.3390/ijms21145103. Int J Mol Sci. 2020. PMID: 32707723 Free PMC article. - Protein-based nanoparticles for therapeutic nucleic acid delivery.
Eweje F, Walsh ML, Ahmad K, Ibrahim V, Alrefai A, Chen J, Chaikof EL. Eweje F, et al. Biomaterials. 2024 Mar;305:122464. doi: 10.1016/j.biomaterials.2023.122464. Epub 2024 Jan 2. Biomaterials. 2024. PMID: 38181574 Free PMC article. Review. - Hybrid Silica-Coated PLGA Nanoparticles for Enhanced Enzyme-Based Therapeutics.
Gustafson KT, Mokhtari N, Manalo EC, Montoya Mira J, Gower A, Yeh YS, Vaidyanathan M, Esener SC, Fischer JM. Gustafson KT, et al. Pharmaceutics. 2022 Dec 31;15(1):143. doi: 10.3390/pharmaceutics15010143. Pharmaceutics. 2022. PMID: 36678770 Free PMC article. - Fractionated photothermal therapy in a murine tumor model: comparison with single dose.
Simón M, Norregaard K, Jørgensen JT, Oddershede LB, Kjaer A. Simón M, et al. Int J Nanomedicine. 2019 Jul 18;14:5369-5379. doi: 10.2147/IJN.S205409. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31409993 Free PMC article. - Zwitterlation mitigates protein bioactivity loss in vitro over PEGylation.
Han Y, Yuan Z, Zhang P, Jiang S. Han Y, et al. Chem Sci. 2018 Sep 14;9(45):8561-8566. doi: 10.1039/c8sc01777h. eCollection 2018 Dec 7. Chem Sci. 2018. PMID: 30568780 Free PMC article.
References
- Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–1458. - PubMed
- Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. - PubMed
- Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol) Langmuir. 2006;22:8178–8185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources