Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways - PubMed (original) (raw)
Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways
Jesmond Dalli et al. FASEB J. 2015 May.
Abstract
Local mediators orchestrate the host response to both sterile and infectious challenge and resolution. Recent evidence demonstrates that maresin sulfido-conjugates actively resolve acute inflammation and promote tissue regeneration. In this report, we investigated self-limited infectious exudates for novel bioactive chemical signals in tissue regeneration and resolution. By use of spleens from Escherichia coli infected mice, self-resolving infectious exudates, human spleens, and blood from patients with sepsis, we identified 2 new families of potent molecules. Characterization of their physical properties and isotope tracking demonstrated that the bioactive structures contained a docosahexaenoate backbone and sulfido-conjugated triene or tetraene double-bond systems. Activated human phagocytes converted 17-hydro(peroxy)-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid to these bioactive molecules. Regeneration of injured planaria was accelerated with nanomolar amounts of 16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19Z-docosahexaenoic acid and 16-cysteinylglycinyl, 17-hydroxy-4Z,7Z,10,12,14,19Z-docosahexaenoic acid (Protectin sulfido-conjugates) or 8-glutathionyl, 7,17-dihydroxy-4Z,9,11,13Z,15E,19Z-docosahexaenoic acid and 8-cysteinylglycinyl, 7,17-dihydroxy-4Z,9,11,13Z,15E,19Z-docosahexaenoic acid (Resolvin sulfido-conjugates). Each protectin and resolvin sulfido-conjugate dose dependently (0.1-10 nM) stimulated human macrophage bacterial phagocytosis, phagolysosomal acidification, and efferocytosis. Together, these results identify 2 novel pathways and provide evidence for structural elucidation of new resolution moduli. These resolvin and protectin conjugates identified in mice and human infected tissues control host responses promoting catabasis.
Keywords: infection; inflammation; leukocytes; resolution.
© FASEB.
Figures
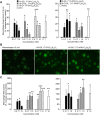
Figure 1.
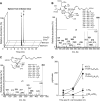
In _E. coli_-infected mouse spleens, identification of novel sulfido-conjugates. Mice were inoculated with E. coli (1 × 105 CFU/mouse) and spleens harvested. Products were extracted using C18 solid-phase columns and investigated by LC-MS-MS (see Materials and Methods). A) MRM chromatograms for the identified sulfido-conjugated products. B and C) MS-MS spectra employed for the identification of (B) 16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid (I) and (C) 16-cysteinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid (II). D) Quantification of sulfido-conjugated mediators in mouse spleen during self-resolving infections (16-GS,17-OH,C22H30O2 = 16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid; 16-CY,17-OH,C22H30O2 = 16-cysteinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid). Results for (A_–_C) are representative of n = 9 mice. Results for (D) are the mean ±
sem
(n = 3 mice per time interval).
Figure 2.
E. coli_-infected mouse exudates gave novel sulfido-conjugates. Mice were inoculated with E. coli (1 × 105 CFU/mouse) and exudates collected 12 or 24 h later. Products were extracted using C18 solid-phase columns and investigated by LC-MS-MS (see Materials and Methods). A) MRM chromatograms for the identified sulfido-conjugated products. B and C) MS-MS spectra employed for the identification of (B) 8-glutathionyl, 7,17-dihydroxy-4Z,9,11,13_Z,15_E_,19_Z_-docosahexaenoic acid and (C)16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid. D) Quantification of sulfido-conjugated mediators in mouse exudates during self-resolving infections (16-GS,17-OH,C22H30O2 = 16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid; 8-GS, 7,17-diOH,C22H29O2 = 8-glutathionyl, 7,17-dihydroxy-4Z,9,11,13_Z_,15_E_,19_Z_-docosahexaenoic acid; 16-CYGL,17-OH,C22H30O2 = 16-cysteinylglycinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid). Results for (A_–_C) are representative of n = 18 mice. Results for (D) are the mean ±
sem
(n = 6 mice per interval). Lavage volume = 5 ml each mouse.
Figure 3.
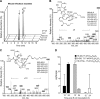
Human spleen identification of novel sulfido-conjugates. Products from human spleens were extracted using C18 solid-phase columns and investigated by LC-MS-MS. A) MRM chromatograms for the identified sulfido-conjugated products. B_–_D) MS-MS spectra employed for the identification of (B) 8-glutathionyl, 7,17-dihydroxy-4Z,9,11,13_Z_,15_E_,19_Z_-docosahexaenoic acid, (C) 16-glutathionyl,17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid, and (D) 16-cysteinyl,17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid. Results are representative of n = 3 human spleens.
Figure 4.
17 lipoxygenation of DHA is precursor to novel sulfido-conjugates with human phagocytes. Human macrophages and neutrophils were incubated 30 min at 37°C with 1 μ_g 17-HpD plus 100 ng zymosan. A) Representative MRM chromatograms for each of the identified 17-series sulfido-conjugates in human macrophages (left panel) and PMN (right panel). B) Representative MS-MS spectra used for the identification of 8-glutathionyl, 7,17-dihydroxy-4Z,9,11,13_Z,15_E_,19_Z_-docosahexaenoic acid (left panel) and 16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid (right panel). C) Quantification of identified sulfido-conjugates. 8-GS, 7,17-diOH-C22H29O2 = 8-glutathionyl, 7,17-dihydroxy-4Z,9,11,13_Z_,15_E_,19_Z_-docosahexaenoic acid; 8-CYGL, 7,17-diOH-C22H29O2 = 8-cysteinylglycinyl, 7,17-dihydroxy-4Z,9,11,13_Z_,15_E_,19_Z_-docosahexaenoic acid; 16-GS,17-OH,C22H30O2 = 16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid; 16-CYGL,17-OH,C22H30O2 = 16-cysteinylglycinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid; 16-CY,17-OH,C22H30O2 = 16-cysteinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid. Results for (A) and (B) are representative of n = 3 healthy donors for each cell type. Results for (C) are the mean (n = 3 healthy donors for each cell type).
Figure 5.
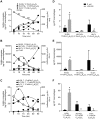
Product-precursor relationships for the novel sulfido-conjugates. Human macrophages (4.5 × 107 cells/ml) were incubated with (A) DHA [37°C (pH 7.45)], (B and C) 17-HpD [37°C (pH 7.45)], and E. coli (1.5 × 108 CFU), and product levels were assessed using LC-MS-MS (see Materials and Methods for details). D_–_F) Human macrophages were incubated with or without GGT inhibitor [Acivicin; 2.5 mM at 37°C (pH 7.45) for 30 min] then (D) DHA or (E and F) 17-HpD [37°C (pH 7.45)] and E. coli (1.5 × 108 CFU). Incubations were quenched; precursor and product levels were assessed by LC-MS-MS. Results are the mean for n = 3 macrophage preparations.*P < 0.05 vs. vehicle macrophages.
Figure 6.
Apoptotic human PMNs produce novel sulfido-conjugates from endogenous DHA and promote E. coli clearance during infection. Apoptotic neutrophil (see Materials and Methods for details) products were obtained and profiled by LC-MS-MS metabololipidomics. A) MRM chromatograms for identified products. B) Representative MS-MS spectrum employed for the identification of 16-cysteinylglycinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid. Results are representative of n = 6 apoptotic PMN preparations. C) Quantification of identified sulfido-conjugates in apoptotic PMN preparations. 8-CYGL, 7,17-diOH-C22H29O2 = 8-cysteinylglycinyl, 7,17-dihydroxy-4Z,9,11,13_Z_,15_E_,19_Z_-docosahexaenoic acid;;16-GS,17-OH,C22H30O2 = 16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid; 16-CYGL,17-OH,C22H30O2 = 16-cysteinylglycinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid. Results are the mean for n = 6 cell preparations. D and E) FVB mice were infected intraperitoneally with E. coli (1 × 105 CFU/mouse). After 4 h, mice were administered saline (E. coli) or apoptotic PMN (apop PMN; 15 × 106 cells per mouse; i.p.). Exudates were then collected 12 h postinfection. D) Exudate macrophage phagocytosis of E. coli was assessed by flow cytometry. E) Bacterial titers present in the peritoneum were measured. Results for (A) and (B) are representative of n = 3 neutrophil preparations. Results for (C) are the mean ±
sem
(n = 3 mice per group). **P ≤ 0.01 and ***P ≤ 0.001 vs. E. coli mice.
Figure 7.
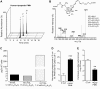
Novel sulfido-conjugates accelerate tissue regeneration in planaria. A_–_C) Planaria were surgically injured, then kept in water containing (B) 16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid plus 16-cysteinylglycinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid (50 nM; each), (C) 8-glutathionyl, 7,17-dihydroxy-4Z,9,11,13_Z_,15_E_,19_Z_-docosahexaenoic acid plus 8-cysteinylglycinyl, 7,17-dihydroxy-4Z,9,11,13_Z_,15_E_,19_Z_-docosahexaenoic acid (50 nM; each), or vehicle (water containing 0.01% EtOH), and tissue regeneration was assessed (see Materials and Methods for details). TRI50, time to 50% regeneration. Results are the mean ±
sem
(n = 4 planaria per group). *P ≤ 0.05 and **P ≤ 0.01 vs. respective surgical injury group.
Figure 8.
17-series sulfido-conjugates display potent anti-inflammatory and proresolving actions with human macrophages. A) Human macrophages (5 × 104 cells per well) were incubated with the indicated concentrations of novel sulfido-conjugated mediators [15 min at 37°C (pH 7.45)] before the addition of fluorescently labeled bacteria (2.5 × 106 cells per well for 40 min at 37°C). Nonphagocytosed cells were washed, extracellular fluorescence was quenched, and phagocytosis assessed using a fluorescence plate reader. B) Human macrophages (5 × 104 cells per well) were incubated with a pH-sensitive fluorophore (30 min at 37°C), then incubated with 10 nM sulfido-conjugated mediators [15 min at 37°C (pH 7.45)] prior to addition of bacteria (2.5 × 106 cells per well for 60 min at 37°C), and assessment of fluorescence was performed using a BZ9000 microscope equipped with a ×20 objective. C) Human macrophages (5 × 104 cells per well) were incubated with the indicated concentrations of the novel sulfido-conjugated products for 10 min at 37°C. Fluorescently labeled apoptotic PMNs (1.5 × 105 cells per well) were then added (40 min at 37°C), and efferocytosis was measured using a fluorescent plate reader. Results for (B) are representative of n = 3 macrophage preparations. Results for (A) and (C) are the mean ±
sem
(n = 3 macrophage preparations). *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 vs. vehicle incubated macrophages.
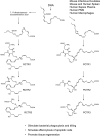
Figure 9.
Proposed biosynthesis of sulfido-protectins and sulfido-resolvins. Structures and pathways are depicted in likely stereochemistry based on biosynthetic evidence (see main text for details). Their stereochemistries as shown are tentative. PCTR1, 16-glutathionyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid; PCTR2, 16-cysteinylglycinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid; PCTR3, 16-cysteinyl, 17-hydroxy-4Z,7Z,10,12,14,19_Z_-docosahexaenoic acid; RCTR1, 8-glutathionyl, 7,17-hydroxy-4Z,9,11,13Z,15E,19Z-docosahexaenoic acid; RCTR2, 8-cysteinylglycinyl, 7,17-hydroxy-4Z,9,11,13Z,15E,19_Z_-docosahexaenoic acid; RCTR3, 8-cysteinyl, 7,17-hydroxy-4Z,9,11,13Z,15E,19_Z_-docosahexaenoic acid.
Similar articles
- Identification and Complete Stereochemical Assignments of the New Resolvin Conjugates in Tissue Regeneration in Human Tissues that Stimulate Proresolving Phagocyte Functions and Tissue Regeneration.
de la Rosa X, Norris PC, Chiang N, Rodriguez AR, Spur BW, Serhan CN. de la Rosa X, et al. Am J Pathol. 2018 Apr;188(4):950-966. doi: 10.1016/j.ajpath.2018.01.004. Am J Pathol. 2018. PMID: 29571326 Free PMC article. - Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions.
Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Serhan CN, et al. J Exp Med. 2009 Jan 16;206(1):15-23. doi: 10.1084/jem.20081880. Epub 2008 Dec 22. J Exp Med. 2009. PMID: 19103881 Free PMC article. - Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes.
Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Serhan CN, et al. J Immunol. 2006 Feb 1;176(3):1848-59. doi: 10.4049/jimmunol.176.3.1848. J Immunol. 2006. PMID: 16424216 - Resolvins and cysteinyl-containing pro-resolving mediators activate resolution of infectious inflammation and tissue regeneration.
Serhan CN, Chiang N. Serhan CN, et al. Prostaglandins Other Lipid Mediat. 2023 Jun;166:106718. doi: 10.1016/j.prostaglandins.2023.106718. Epub 2023 Feb 21. Prostaglandins Other Lipid Mediat. 2023. PMID: 36813255 Free PMC article. Review. - New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration.
Serhan CN, Chiang N, Dalli J. Serhan CN, et al. Mol Aspects Med. 2018 Dec;64:1-17. doi: 10.1016/j.mam.2017.08.002. Epub 2017 Sep 1. Mol Aspects Med. 2018. PMID: 28802833 Free PMC article. Review.
Cited by
- Maresin-1 Prevents Liver Fibrosis by Targeting Nrf2 and NF-κB, Reducing Oxidative Stress and Inflammation.
Rodríguez MJ, Sabaj M, Tolosa G, Herrera Vielma F, Zúñiga MJ, González DR, Zúñiga-Hernández J. Rodríguez MJ, et al. Cells. 2021 Dec 3;10(12):3406. doi: 10.3390/cells10123406. Cells. 2021. PMID: 34943914 Free PMC article. - Anti-inflammatory interventions-what has worked, not worked, and what may work in the future.
Fattahi F, Ward PA. Fattahi F, et al. Transl Res. 2016 Jan;167(1):1-6. doi: 10.1016/j.trsl.2015.08.003. Epub 2015 Aug 14. Transl Res. 2016. PMID: 26323016 Free PMC article. - Specialized Proresolving Mediators Rescue Infant Mice from Lethal Citrobacter rodentium Infection and Promote Immunity against Reinfection.
Diaz LA, Altman NH, Khan WN, Serhan CN, Adkins B. Diaz LA, et al. Infect Immun. 2017 Sep 20;85(10):e00464-17. doi: 10.1128/IAI.00464-17. Print 2017 Oct. Infect Immun. 2017. PMID: 28694292 Free PMC article. - The Resolution Approach to Cystic Fibrosis Inflammation.
Recchiuti A, Patruno S, Plebani R, Romano M. Recchiuti A, et al. Front Pharmacol. 2020 Jul 29;11:1129. doi: 10.3389/fphar.2020.01129. eCollection 2020. Front Pharmacol. 2020. PMID: 32848748 Free PMC article. Review. - Can Specialized Pro-resolving Mediators Deliver Benefit Originally Expected from Fish Oil?
Rosenthal MD, Patel J, Staton K, Martindale RG, Moore FA, Upchurch GR Jr. Rosenthal MD, et al. Curr Gastroenterol Rep. 2018 Aug 4;20(9):40. doi: 10.1007/s11894-018-0647-4. Curr Gastroenterol Rep. 2018. PMID: 30078085 Review.
References
- Avicenna, adapted by L. Bakhtiar (1999) The Canon of Medicine (al-Qanun fi'l-tibb), G. B. I. W., Incorporated, Chicago
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources