Mitochondrial uncoupling proteins and energy metabolism - PubMed (original) (raw)
Review
Mitochondrial uncoupling proteins and energy metabolism
Rosa A Busiello et al. Front Physiol. 2015.
Abstract
Understanding the metabolic factors that contribute to energy metabolism (EM) is critical for the development of new treatments for obesity and related diseases. Mitochondrial oxidative phosphorylation is not perfectly coupled to ATP synthesis, and the process of proton-leak plays a crucial role. Proton-leak accounts for a significant part of the resting metabolic rate (RMR) and therefore enhancement of this process represents a potential target for obesity treatment. Since their discovery, uncoupling proteins have stimulated great interest due to their involvement in mitochondrial-inducible proton-leak. Despite the widely accepted uncoupling/thermogenic effect of uncoupling protein one (UCP1), which was the first in this family to be discovered, the reactions catalyzed by its homolog UCP3 and the physiological role remain under debate. This review provides an overview of the role played by UCP1 and UCP3 in mitochondrial uncoupling/functionality as well as EM and suggests that they are a potential therapeutic target for treating obesity and its related diseases such as type II diabetes mellitus.
Keywords: energy metabolism; mitochondria; obesity; proton-leak; uncoupling protein.
Figures
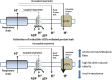
Figure 1
Schematic representation of proton-leak and of the proposed role of UCPs in influencing energy metabolism.
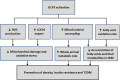
Figure 2
Schematic diagram that illustrates how the reactions influenced by UCP3 could be redirected to prevent or treat obesity, insulin resistance, and type 2 diabetes (T2DM).
Similar articles
- UCP3 and its putative function: consistencies and controversies.
Harper ME, Dent RM, Bezaire V, Antoniou A, Gauthier A, Monemdjou S, McPherson R. Harper ME, et al. Biochem Soc Trans. 2001 Nov;29(Pt 6):768-73. doi: 10.1042/bst0290768. Biochem Soc Trans. 2001. PMID: 11709072 Review. - Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism.
Boss O, Hagen T, Lowell BB. Boss O, et al. Diabetes. 2000 Feb;49(2):143-56. doi: 10.2337/diabetes.49.2.143. Diabetes. 2000. PMID: 10868929 Review. - Mitochondrial uncoupling, ROS generation and cardioprotection.
Cadenas S. Cadenas S. Biochim Biophys Acta Bioenerg. 2018 Sep;1859(9):940-950. doi: 10.1016/j.bbabio.2018.05.019. Epub 2018 May 31. Biochim Biophys Acta Bioenerg. 2018. PMID: 29859845 Review. - Mitochondrial proton leak: a role for uncoupling proteins 2 and 3?
Porter RK. Porter RK. Biochim Biophys Acta. 2001 Mar 1;1504(1):120-7. doi: 10.1016/s0005-2728(00)00246-2. Biochim Biophys Acta. 2001. PMID: 11239489 Review. - Cold tolerance of UCP1-ablated mice: a skeletal muscle mitochondria switch toward lipid oxidation with marked UCP3 up-regulation not associated with increased basal, fatty acid- or ROS-induced uncoupling or enhanced GDP effects.
Shabalina IG, Hoeks J, Kramarova TV, Schrauwen P, Cannon B, Nedergaard J. Shabalina IG, et al. Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):968-80. doi: 10.1016/j.bbabio.2010.02.033. Epub 2010 Mar 19. Biochim Biophys Acta. 2010. PMID: 20227385
Cited by
- Metabolic Adaptation in Heart Failure and the Role of Ketone Bodies as Biomarkers.
Foster MW, Riley JM, Kaki PC, Al Soueidy A, Aligholiazadeh E, Rame JE. Foster MW, et al. Curr Heart Fail Rep. 2024 Oct;21(5):498-503. doi: 10.1007/s11897-024-00678-6. Epub 2024 Sep 7. Curr Heart Fail Rep. 2024. PMID: 39242479 Free PMC article. Review. - Obesity history, physical exam, laboratory, body composition, and energy expenditure: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022.
Burridge K, Christensen SM, Golden A, Ingersoll AB, Tondt J, Bays HE. Burridge K, et al. Obes Pillars. 2022 Jan 10;1:100007. doi: 10.1016/j.obpill.2021.100007. eCollection 2022 Mar. Obes Pillars. 2022. PMID: 37990700 Free PMC article. - DJ-1 Deficiency Protects Hepatic Steatosis by Enhancing Fatty Acid Oxidation in Mice.
Xu M, Wu H, Li M, Wen Y, Yu C, Xia L, Xia Q, Kong X. Xu M, et al. Int J Biol Sci. 2018 Oct 20;14(13):1892-1900. doi: 10.7150/ijbs.28620. eCollection 2018. Int J Biol Sci. 2018. PMID: 30443192 Free PMC article. - The DNA Repair Protein OGG1 Protects Against Obesity by Altering Mitochondrial Energetics in White Adipose Tissue.
Komakula SSB, Tumova J, Kumaraswamy D, Burchat N, Vartanian V, Ye H, Dobrzyn A, Lloyd RS, Sampath H. Komakula SSB, et al. Sci Rep. 2018 Oct 5;8(1):14886. doi: 10.1038/s41598-018-33151-1. Sci Rep. 2018. PMID: 30291284 Free PMC article. - Limited Oxidative Stress Favors Resistance to Skeletal Muscle Atrophy in Hibernating Brown Bears (Ursus Arctos).
Chazarin B, Ziemianin A, Evans AL, Meugnier E, Loizon E, Chery I, Arnemo JM, Swenson JE, Gauquelin-Koch G, Simon C, Blanc S, Lefai E, Bertile F. Chazarin B, et al. Antioxidants (Basel). 2019 Aug 22;8(9):334. doi: 10.3390/antiox8090334. Antioxidants (Basel). 2019. PMID: 31443506 Free PMC article.
References
- Adams S. H., Hoppel C. L., Lok K. H., Zhao L., Wong S. W., Minkler P. E., et al. . (2009). Plasma acylcarnitine profiles suggestincomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycleactivity in type 2 diabetic African-American women. J. Nutr. 139, 1073–1081. 10.3945/jn.108.103754 - DOI - PMC - PubMed
- Argyropoulos G., Brown A. M., Willi S. M., Zhu J., He Y., Reitman M., et al. . (1998). Effects of mutations in the human uncoupling protein 3 gene on the respiratory quotient and fat oxidation in severe obesity and type 2 diabetes. J. Clin. Invest. 102, 1345–1351. 10.1172/JCI4115 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources