Myofascial Trigger Points Then and Now: A Historical and Scientific Perspective - PubMed (original) (raw)
Review
Myofascial Trigger Points Then and Now: A Historical and Scientific Perspective
Jay P Shah et al. PM R. 2015 Jul.
Abstract
The intent of this article is to discuss the evolving role of the myofascial trigger point (MTrP) in myofascial pain syndrome (MPS) from both a historical and scientific perspective. MTrPs are hard, discrete, palpable nodules in a taut band of skeletal muscle that may be spontaneously painful (i.e., active) or painful only on compression (i.e., latent). MPS is a term used to describe a pain condition that can be acute or, more commonly, chronic and involves the muscle and its surrounding connective tissue (e.g. fascia). According to Travell and Simons, MTrPs are central to the syndrome-but are they necessary? Although the clinical study of muscle pain and MTrPs has proliferated over the past two centuries, the scientific literature often seems disjointed and confusing. Unfortunately, much of the terminology, theories, concepts, and diagnostic criteria are inconsistent, incomplete, or controversial. To address these deficiencies, investigators have recently applied clinical, imaging (of skeletal muscle and brain), and biochemical analyses to systematically and objectively study the MTrP and its role in MPS. Data suggest that the soft tissue milieu around the MTrP, neurogenic inflammation, sensitization, and limbic system dysfunction may all play a role in the initiation, amplification, and perpetuation of MPS. The authors chronicle the advances that have led to the current understanding of MTrP pathophysiology and its relationship to MPS, and review the contributions of clinicians and researchers who have influenced and expanded our contemporary level of clinical knowledge and practice.
Published by Elsevier Inc.
Figures
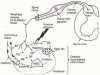
Figure 1
Schematic of a trigger point complex. A trigger point complex in a taut band of muscle is composed of multiple contraction knots (Adapted from Simons, D.G., Travell, J.G. Myofascial Pain and Dysfunction: The Trigger Point Manual, vol. 1; second ed., and Användare: Chrizz., In: Shah, J. P. and E. A. Gilliams (2008). “Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome.”
J Bodyw Mov Ther
12(4): 371-384, used with permission.)
Figure 2
Simultaneous 2D gray-scale and color variance imaging. (A and B) Normal upper trapezius muscle. The normal muscle appears isoechoic and has uniform color variance (TIS=0). (C and D) Muscle with a palpable MTrP. A hypoechoic region and a well-defined focal decrease of color variance indicating a localized stiffer region is visible (TIS=1). (E and F) Muscle with a palpable MTrP. Multiple hypoechoic regions and multiple focal nodules are visible (TIS=2). Abbreviation: TIS, tissue imaging score. (In: Sikdar S, Shah JP, Gebreab T, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. Nov 2009;90(11):1829-1838, used with permission).
Figure 3
Neurogenic inflammation. In the presence of persistent nociceptive bombardment, the dorsal root ganglion will release substance P and CGRP (not shown) antidromically into the peripheral tissue. The peripheral secretion of these substances can lead to a cascade of events, including the degranulation of local mast cells, local vasodilation, plasma extravasation and the development of a sensitizing biochemical soup. This process of neurogenic inflammation leads to the enhanced release of endogenous substances, such as bradykinin, serotonin, norepinephrine, nerve growth factor, and adenosine. (In: Everett, T., Dennis M., Ricketts E,. eds. Physiotherapy in mental health : a practical approach. Oxford UK: Butterworth/Heinemann; 1995: 102-126, used with permission).
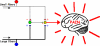
Figure 4
Gate Control Theory. Pain stimulation activates small nerve fibers. As a result, the fibers send input to the neurons to block the inhibitory interneuron (I), which is now unable to block the output of the projection neuron (P) that connects with the brain. Since the excitatory gate is open, pain is perceived. Non-painful stimulation activates large nerve fibers primarily. As a result, the projection neuron (P) and inhibitory interneuron (I) is activated. However, because the inhibitory interneuron blocks the signal in the projection neuron (P) that connects to the brain, the excitatory gate is closed, and no pain is perceived. Without any stimulation, neither large nor small nerve fibers are activated. The inhibitory interneuron (I) blocks the signal in the projection neuron (P) that connects to the brain. The excitatory gate is closed and no pain is perceived. (Accessed from
https://faculty.washington.edu/chudler/pain.html
).
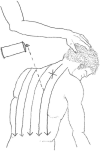
Figure 5
Spray and Stretch Application. The lower posterior muscles of the upper back are stretched while the spray is applied in a downward motion, from the patient's neck to the referred pain region. (Drawn from Ferguson, L. W. and R. Gerwin (2005). Clinical mastery in the treatment of myofascial pain. Philadelphia, Lippincott Williams & Wilkins.).
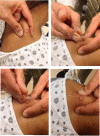
Figure 6
Dry Needling. A series of images are shown in which the MTrP is identified, the needled is inserted in the MTrP using a swift tap, the muscle and surrounding fascia are probed with an up and down motion of the needle in a clockwise direction, and the needle is left in place for 1-2 minutes for full therapeutic benefit.
Similar articles
- Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome.
Shah JP, Gilliams EA. Shah JP, et al. J Bodyw Mov Ther. 2008 Oct;12(4):371-384. doi: 10.1016/j.jbmt.2008.06.006. Epub 2008 Aug 13. J Bodyw Mov Ther. 2008. PMID: 19083696 Review. - Re-Examining Myofascial Pain Syndrome: Toward Biomarker Development and Mechanism-Based Diagnostic Criteria.
Duarte FCK, West DWD, Linde LD, Hassan S, Kumbhare DA. Duarte FCK, et al. Curr Rheumatol Rep. 2021 Jul 8;23(8):69. doi: 10.1007/s11926-021-01024-8. Curr Rheumatol Rep. 2021. PMID: 34236529 Review. - Dry Needling on the Infraspinatus Latent and Active Myofascial Trigger Points in Older Adults With Nonspecific Shoulder Pain: A Randomized Clinical Trial.
Calvo-Lobo C, Pacheco-da-Costa S, Martínez-Martínez J, Rodríguez-Sanz D, Cuesta-Álvaro P, López-López D. Calvo-Lobo C, et al. J Geriatr Phys Ther. 2018 Jan/Mar;41(1):1-13. doi: 10.1519/JPT.0000000000000079. J Geriatr Phys Ther. 2018. PMID: 26760574 Free PMC article. Clinical Trial. - Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue.
Sikdar S, Shah JP, Gebreab T, Yen RH, Gilliams E, Danoff J, Gerber LH. Sikdar S, et al. Arch Phys Med Rehabil. 2009 Nov;90(11):1829-38. doi: 10.1016/j.apmr.2009.04.015. Arch Phys Med Rehabil. 2009. PMID: 19887205 Free PMC article. - Myofascial trigger points in migraine and tension-type headache.
Do TP, Heldarskard GF, Kolding LT, Hvedstrup J, Schytz HW. Do TP, et al. J Headache Pain. 2018 Sep 10;19(1):84. doi: 10.1186/s10194-018-0913-8. J Headache Pain. 2018. PMID: 30203398 Free PMC article. Review.
Cited by
- Current advances in the treatment of myofascial pain syndrome with trigger point injections: A review.
Anwar N, Wei X, Jie Y, Hongbo Z, Jin H, Zhu Z. Anwar N, et al. Medicine (Baltimore). 2024 Oct 4;103(40):e39885. doi: 10.1097/MD.0000000000039885. Medicine (Baltimore). 2024. PMID: 39465697 Free PMC article. Review. - The Case for Comorbid Myofascial Pain-A Qualitative Review.
Vulfsons S, Minerbi A. Vulfsons S, et al. Int J Environ Res Public Health. 2020 Jul 17;17(14):5188. doi: 10.3390/ijerph17145188. Int J Environ Res Public Health. 2020. PMID: 32709141 Free PMC article. Review. - Quantitative proteomics analysis to identify biomarkers of chronic myofascial pain and therapeutic targets of dry needling in a rat model of myofascial trigger points.
Li LH, Huang QM, Barbero M, Liu L, Nguyen TT, Beretta-Piccoli M, Xu AL, Ji LJ. Li LH, et al. J Pain Res. 2019 Jan 7;12:283-298. doi: 10.2147/JPR.S185916. eCollection 2019. J Pain Res. 2019. PMID: 30662282 Free PMC article. - A randomized clinical trial comparing non-thrust manipulation with segmental and distal dry needling on pain, disability, and rate of recovery for patients with non-specific low back pain.
Griswold D, Gargano F, Learman KE. Griswold D, et al. J Man Manip Ther. 2019 Jul;27(3):141-151. doi: 10.1080/10669817.2019.1574389. Epub 2019 Feb 9. J Man Manip Ther. 2019. PMID: 30935327 Free PMC article. Clinical Trial. - Comparison of ozone and lidocaine injection efficacy vs dry needling in myofascial pain syndrome patients.
Raeissadat SA, Rayegani SM, Sadeghi F, Rahimi-Dehgolan S. Raeissadat SA, et al. J Pain Res. 2018 Jun 29;11:1273-1279. doi: 10.2147/JPR.S164629. eCollection 2018. J Pain Res. 2018. PMID: 29988746 Free PMC article.
References
- Gerwin RD. Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001 Oct;5(5):412–420. - PubMed
- Fricton JR, Kroening R, Haley D, Siegert R. Myofascial pain syndrome of the head and neck: a review of clinical characteristics of 164 patients. Oral Surg Oral Med Oral Pathol. 1985 Dec;60(6):615–623. - PubMed
- Bennett MJ, McLaughlin S, Anderson T, McDicken WN. Error analysis of ultrasonic tissue doppler velocity estimation techniques for quantification of velocity and strain. Ultrasound in Medicine & Biology. 2007;33(1):74–81. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous